Earth's resources -> boiling point
Boiling Point
The boiling point of a substance is the temperature at which it changes from a liquid to a gas. It is an important physical property that can vary depending on the surrounding pressure. The boiling point is a useful characteristic for identifying and classifying different substances.
Factors Affecting Boiling Point
The boiling point of a substance is influenced by several factors:
- Molecular Structure: The type and arrangement of molecules in a substance can affect its boiling point. Generally, substances with stronger intermolecular forces have higher boiling points.
- Pressure: As the pressure increases, the boiling point of a substance also increases. This is why the boiling point of water is lower at higher altitudes where the atmospheric pressure is lower.
- Purity: Impurities in a substance can lower its boiling point. Pure substances typically have higher boiling points than mixtures.
Examples of Boiling Points
Here are the boiling points of some common substances at standard atmospheric pressure (1 atm or 101.3 kPa):
| Substance | Boiling Point (°C) |
|---|---|
| Water | 100°C |
| Ethanol | 78.37°C |
| Acetone | 56.2°C |
| Nitrogen | -196°C |
Study Guide
When studying boiling point, consider the following key points:
- Understand the concept of boiling point and how it differs from melting point.
- Learn how different factors such as molecular structure, pressure, and purity can affect boiling points.
- Practice identifying substances based on their boiling points.
- Explore real-life examples of the importance of boiling points, such as in cooking and industrial processes.
◂Science Worksheets and Study Guides First Grade. Earth's resources
Study Guide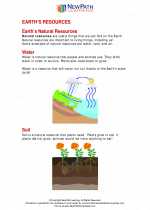 Earth's resources
Earth's resources  Worksheet/Answer key
Worksheet/Answer key Earth's resources
Earth's resources  Worksheet/Answer key
Worksheet/Answer key Earth's resources
Earth's resources  Worksheet/Answer key
Worksheet/Answer key Earth's resources
Earth's resources  Vocabulary/Answer key
Vocabulary/Answer key Earth's resources
Earth's resources  Vocabulary/Answer key
Vocabulary/Answer key Earth's resources
Earth's resources 

 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key

The resources above cover the following skills:
EARTH AND SPACE SCIENCE (NGSS)
Earth’s Place in the Universe
Students who demonstrate understanding can:
Use observations of the sun, moon, and stars to describe patterns that can be predicted.
Make observations at different times of year to relate the amount of daylight to the time of year.