Atomic Mass
Atomic mass is a property of an atom that represents the total mass of the protons, neutrons, and electrons in an atom. It is typically expressed in atomic mass units (amu).
Protons and Neutrons
Protons and neutrons are located in the nucleus of an atom. Protons have a positive charge, while neutrons have no charge. The total number of protons and neutrons in an atom is known as the mass number.
Electrons
Electrons have a much smaller mass compared to protons and neutrons, so they are often not included in the calculation of atomic mass. However, in some cases, the mass of electrons may be considered when determining the atomic mass of an atom.
Calculating Atomic Mass
The atomic mass of an atom is calculated by adding the total number of protons and neutrons. Since electrons have a very small mass compared to protons and neutrons, they are often not included in the calculation.
Study Guide
.◂Science Worksheets and Study Guides First Grade. Human body

 Activity Lesson
Activity Lesson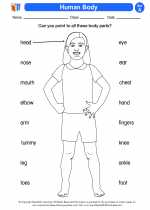
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key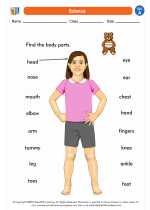
 Worksheet/Answer key
Worksheet/Answer key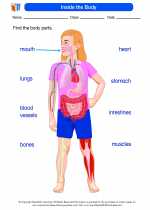
 Vocabulary/Answer key
Vocabulary/Answer key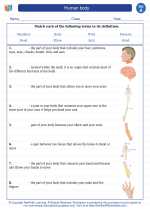
 Vocabulary/Answer key
Vocabulary/Answer key
