Carbonate
Carbonate is a salt or ester of carbonic acid. It is a compound made up of carbon, oxygen, and other elements, such as calcium, magnesium, or iron. Carbonates are commonly found in nature, especially in rocks and minerals.
Properties of Carbonates:
- Most carbonates are insoluble in water.
- They react with acids to produce carbon dioxide gas.
- Many carbonates are important mineral resources, such as limestone, marble, and chalk.
Examples of Carbonates:
Some common examples of carbonates include:
- Calcium carbonate (CaCO3) - found in limestone, marble, and chalk.
- Sodium carbonate (Na2CO3) - known as washing soda.
- Potassium carbonate (K2CO3) - used in the production of glass.
Study Guide:
When studying carbonates, it's important to understand their chemical composition, properties, and practical applications. Here are some key points to focus on:
- Define what a carbonate is and identify its chemical formula.
- Learn about the solubility of carbonates in water and their reaction with acids.
- Explore the different types of carbonates and their uses in everyday life.
- Examine the role of carbonates in the formation of rocks and minerals.
- Conduct experiments to observe the reactions of carbonates with acids and the production of carbon dioxide gas.
Understanding carbonates is important in the fields of geology, chemistry, and environmental science. By grasping the properties and uses of carbonates, you can gain a deeper appreciation for the role of these compounds in the natural world and human activities.
[Carbonate] Related Worksheets and Study Guides:
.◂Science Worksheets and Study Guides First Grade. Human body
Study Guide Human body
Human body  Activity Lesson
Activity Lesson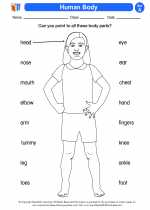 Human Body
Human Body  Worksheet/Answer key
Worksheet/Answer key Human body
Human body  Worksheet/Answer key
Worksheet/Answer key Human body
Human body  Worksheet/Answer key
Worksheet/Answer key Human body
Human body  Worksheet/Answer key
Worksheet/Answer key My Senses
My Senses  Worksheet/Answer key
Worksheet/Answer key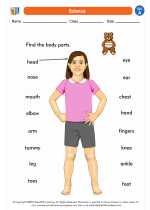 Human Body
Human Body  Worksheet/Answer key
Worksheet/Answer key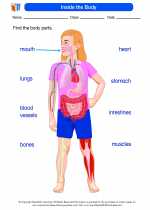 Inside the Body
Inside the Body  Vocabulary/Answer key
Vocabulary/Answer key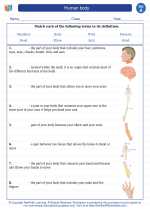 Human body
Human body  Vocabulary/Answer key
Vocabulary/Answer key Human body
Human body 

 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key

The resources above cover the following skills:
LIFE SCIENCE
From Molecules to Organisms: Structures and Processes
Design a solution to a human problem by using materials to imitate how plants and/or animals use their external parts to help them survive, grow, and meet their needs (e.g., outerwear imitating animal furs for insulation, gear mimicking tree bark or shells for protection).