States of Matter
The states of matter are the distinct forms that different phases of matter take on. There are three primary states of matter: solid, liquid, and gas. Each state has its own unique properties.
Solid
A solid has a definite shape and volume. The particles in a solid are closely packed and vibrate in place. They have a strong attraction to each other, which keeps them in a fixed position. Examples of solids include ice, wood, and metal.
Liquid
A liquid has a definite volume but takes the shape of its container. The particles in a liquid are still close together, but they are able to move around each other. They have weaker attraction compared to solids. Examples of liquids include water, milk, and oil.
Gas
A gas has no definite shape or volume. The particles in a gas are far apart and move freely. They have very weak attraction to each other. Examples of gases include air, steam, and helium.
Changes in States of Matter
Matter can change from one state to another through heating or cooling. These changes are known as phase transitions. For example, when water is heated, it changes from a solid (ice) to a liquid (water) and then to a gas (steam).
Review Questions
.◂Science Worksheets and Study Guides Second Grade. Comparing matter

 Activity Lesson
Activity Lesson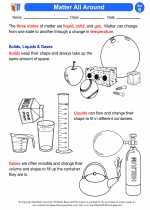
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
