Topic: Waxes
Waxes are a type of organic compound that are hydrophobic (repel water) and have a variety of uses in everyday life.
Structure of Waxes
Waxes are composed of long-chain fatty acids and alcohols. The main component of waxes is a long-chain saturated hydrocarbon, typically containing 14-30 carbon atoms. The hydrophobic nature of waxes is due to their nonpolar structure.
Functions of Waxes
Waxes serve several important functions in living organisms and inanimate objects:
- Waterproofing: Waxes provide a waterproof coating on the surfaces of leaves, fruits, and animal fur or feathers, helping to prevent water loss and protect against environmental damage.
- Protection: In plants, waxes act as a protective barrier against pathogens, UV radiation, and herbivores. In animals, waxes can help to repel water and provide insulation.
- Industrial Uses: Waxes are used in various industrial applications, such as in the production of candles, polishes, coatings, and as a component of cosmetic products.
Study Guide
- Chemical Composition: What are the main components of waxes, and how do they contribute to the hydrophobic nature of waxes?
- Functions: Discuss the role of waxes in waterproofing and protection in living organisms. Provide examples of how waxes are used in industrial applications.
- Comparisons: Compare and contrast the properties of waxes with other hydrophobic substances, such as oils and fats.
Understanding the structure and functions of waxes is important for understanding their diverse roles in nature and human applications.
.◂Science Worksheets and Study Guides Second Grade. Comparing matter
Study Guide Comparing matter
Comparing matter  Activity Lesson
Activity Lesson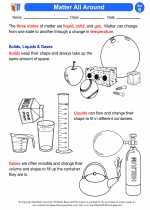 Matter All Around
Matter All Around  Worksheet/Answer key
Worksheet/Answer key Comparing matter
Comparing matter  Worksheet/Answer key
Worksheet/Answer key Comparing matter
Comparing matter  Worksheet/Answer key
Worksheet/Answer key Comparing matter
Comparing matter  Vocabulary/Answer key
Vocabulary/Answer key Comparing matter
Comparing matter 

 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key

The resources above cover the following skills:
PHYSICAL SCIENCE (NGSS)
Matter and its Interactions
Students who demonstrate understanding can:
Construct an argument with evidence that some changes caused by heating or cooling can be reversed and some cannot.