Methane
Methane is a chemical compound with the molecular formula CH4. It is a colorless, odorless gas and is the simplest alkane and the main component of natural gas. Here are some key points to understand about methane:
Properties of Methane:
- Methane is a non-polar molecule, which means it does not have a positive or negative end, and it is not soluble in water.
- It is lighter than air and has a specific gravity of 0.554, which means it will float in the atmosphere.
- Methane is flammable and can form explosive mixtures with air when it reaches a concentration of 5-15% in the air.
Sources of Methane:
Methane is produced through both natural and human activities. Some of the main sources include:
- Natural sources such as wetlands, termites, oceans, and volcanoes.
- Human activities including the production and transport of coal, oil, and natural gas, as well as livestock farming and waste management.
Uses of Methane:
Methane has various applications, including:
- As a fuel for heating and cooking.
- As a feedstock in the production of chemicals such as ammonia, methanol, and hydrogen.
- As a source of energy in power generation.
Environmental Impact:
Methane is a potent greenhouse gas, and its release into the atmosphere contributes to global warming and climate change. It has a much higher global warming potential than carbon dioxide over a 20-year period, although it has a shorter atmospheric lifetime.
Study Guide Questions:
- What is the molecular formula of methane?
- What are the natural sources of methane?
- What are the properties of methane that make it a useful fuel?
- How does the release of methane impact the environment?
- What are the main human activities that contribute to methane emissions?
◂Science Worksheets and Study Guides Second Grade. Mammals and birds
Study Guide Mammals and birds
Mammals and birds  Activity Lesson
Activity Lesson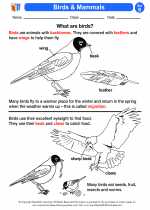 Birds & Mammals
Birds & Mammals  Worksheet/Answer key
Worksheet/Answer key Mammals and birds
Mammals and birds  Worksheet/Answer key
Worksheet/Answer key Mammals and birds
Mammals and birds  Worksheet/Answer key
Worksheet/Answer key Mammals and birds
Mammals and birds  Worksheet/Answer key
Worksheet/Answer key Mammals and birds
Mammals and birds  Vocabulary/Answer key
Vocabulary/Answer key Mammals and birds
Mammals and birds 

 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key

The resources above cover the following skills:
LIFE SCIENCE (NGSS)
Biological Evolution: Unity and Diversity
Students who demonstrate understanding can:
Make observations of plants and animals to compare the diversity of life in different habitats[Clarification Statement: Emphasis is on the diversity of living things in each of a variety of different habitats.] [Assessment Boundary: Assessment does not include specific animal and plant names in specific habitats.]