The sun and earth -> chemical reaction
Chemical Reactions
What is a Chemical Reaction?
A chemical reaction is a process in which one or more substances (reactants) are converted into different substances (products). During a chemical reaction, bonds between atoms are broken and new bonds are formed, resulting in the creation of new substances.
Key Terms
- Reactants: The substances that undergo a chemical change in a reaction.
- Products: The new substances that are formed as a result of a chemical reaction.
- Chemical Equation: A representation of a chemical reaction using chemical formulas and symbols.
- Endothermic Reaction: A reaction that absorbs heat from its surroundings.
- Exothermic Reaction: A reaction that releases heat to its surroundings.
Types of Chemical Reactions
Chemical reactions can be classified into several types, including:
- Synthesis Reaction: Two or more substances combine to form a new compound.
- Decomposition Reaction: A compound breaks down into two or more simpler substances.
- Single Replacement Reaction: An element replaces another element in a compound.
- Double Replacement Reaction: The cations and anions of two different compounds switch places, forming two new compounds.
- Combustion Reaction: A substance reacts with oxygen to produce heat and light.
Factors Affecting Chemical Reactions
Several factors can influence the rate and outcome of chemical reactions, including:
- Temperature: Increasing temperature generally speeds up reactions by providing more energy for particles to collide.
- Concentration of Reactants: Higher concentrations of reactants can lead to more frequent collisions and faster reactions.
- Surface Area: Increasing the surface area of solid reactants can accelerate reactions by exposing more particles to potential collisions.
- Catalysts: Substances that can increase the rate of a reaction without being consumed in the process.
Examples of Chemical Reactions
Common examples of chemical reactions include:
- Combustion of wood or fuel
- Rusting of iron
- Baking a cake
- Photosynthesis in plants
- Digestion of food in the body
Conclusion
Understanding chemical reactions is essential in comprehending many natural processes and human-made products. By grasping the key concepts and types of chemical reactions, you can appreciate the transformations that occur in the world around you.
[Chemical Reaction] Related Worksheets and Study Guides:
.◂Science Worksheets and Study Guides Second Grade. The sun and earth
Study Guide The sun and earth
The sun and earth  Activity Lesson
Activity Lesson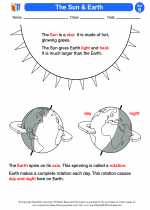 The Sun & Earth
The Sun & Earth  Worksheet/Answer key
Worksheet/Answer key The sun and earth
The sun and earth  Worksheet/Answer key
Worksheet/Answer key The sun and earth
The sun and earth  Worksheet/Answer key
Worksheet/Answer key The sun and earth
The sun and earth  Worksheet/Answer key
Worksheet/Answer key The Sun and Earth
The Sun and Earth  Vocabulary/Answer key
Vocabulary/Answer key The sun and earth
The sun and earth 

 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key

The resources above cover the following skills:
Concepts of Earth Science: A student should understand and be able to apply the concepts, processes, theories, models, evidence, and systems of earth and space sciences. A student who meets the content standard should:
Develop an understanding of the cyclical changes controlled by energy from the sun and by Earth's position and motion in our solar system.