Silver
Silver is a chemical element with the symbol Ag and atomic number 47. It is a soft, white, lustrous transition metal. Silver has been used for thousands of years for various purposes, including currency, jewelry, and as a component in electronics and photography.
Physical Properties
- Symbol: Ag
- Atomic Number: 47
- Color: Silver-white
- State: Solid
- Melting Point: 961.78°C
- Boiling Point: 2162°C
Chemical Properties
Silver is a relatively unreactive metal. It does not react with oxygen or water at normal temperatures, but it does react with sulfur compounds in the air, forming a layer of tarnish on the surface.
Uses of Silver
Silver has a wide range of uses, including:
- Currency: Silver has been used as a form of currency in many cultures throughout history.
- Jewelry and Decorations: Silver is prized for its lustrous appearance and is commonly used in jewelry and decorative items.
- Electronics: Silver is an excellent conductor of electricity, making it valuable for use in electronics and electrical contacts.
- Photography: Silver compounds are used in traditional black-and-white photography.
- Medicine: Silver has antimicrobial properties and is used in some medical applications, such as wound dressings and catheters.
Environmental Impact
Silver is a valuable and relatively rare metal, and its mining and extraction can have environmental impacts. Efforts are being made to improve the sustainability of silver mining and reduce its environmental footprint.
Fun Fact
Silver is the best conductor of electricity of all the elements.
Study Guide
- What is the chemical symbol for silver? Ag
- What is the atomic number of silver? 47
- What is the color of silver? Silver-white
- What is the melting point of silver? 961.78°C
- What are some common uses of silver? Currency, jewelry, electronics, photography, and medicine
- What is a unique property of silver related to its use in electronics? It is an excellent conductor of electricity
◂Science Worksheets and Study Guides Second Grade. The sun and earth

 Activity Lesson
Activity Lesson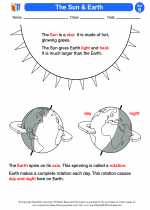
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
