Zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. It is a bluish-white metal and is found in group 12 of the periodic table.
Physical Properties of Zinc
- Atomic number: 30
- Atomic mass: 65.38
- Melting point: 419.5°C
- Boiling point: 907°C
- Density: 7.14 g/cm3
Chemical Properties of Zinc
Zinc is a moderately reactive metal. It readily forms compounds with other elements, and reacts with acids, alkalis, and other non-metals. It does not react with water. Zinc is known for its ability to undergo galvanization, a process of applying a protective zinc coating to steel or iron to prevent rusting.
Uses of Zinc
Zinc is commonly used in a variety of applications, including:
- Galvanization of steel
- Production of brass and bronze
- As a component in batteries
- In the production of zinc oxide, which is used in rubber manufacturing, ceramics, and as a pigment in paints
- In dietary supplements and as an ingredient in various pharmaceutical products
Study Guide
To understand zinc better, consider the following questions:
- What are the physical properties of zinc? Describe its appearance, melting point, boiling point, and density.
- How does zinc react with acids, alkalis, and other non-metals? Provide examples of zinc compounds.
- What is galvanization, and how does zinc play a role in this process?
- What are the common uses of zinc in everyday life and industry?
- How is zinc important in biological systems and human health?
By exploring these questions, you can gain a comprehensive understanding of the properties and uses of zinc.
.◂Science Worksheets and Study Guides Second Grade. The sun and earth
Study Guide The sun and earth
The sun and earth  Activity Lesson
Activity Lesson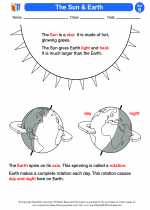 The Sun & Earth
The Sun & Earth  Worksheet/Answer key
Worksheet/Answer key The sun and earth
The sun and earth  Worksheet/Answer key
Worksheet/Answer key The sun and earth
The sun and earth  Worksheet/Answer key
Worksheet/Answer key The sun and earth
The sun and earth  Worksheet/Answer key
Worksheet/Answer key The Sun and Earth
The Sun and Earth  Vocabulary/Answer key
Vocabulary/Answer key The sun and earth
The sun and earth 

 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key

The resources above cover the following skills:
Concepts of Earth Science: A student should understand and be able to apply the concepts, processes, theories, models, evidence, and systems of earth and space sciences. A student who meets the content standard should:
Develop an understanding of the cyclical changes controlled by energy from the sun and by Earth's position and motion in our solar system.