Properties of Potassium Nitrate
Potassium nitrate is a colorless and odorless crystalline solid that is soluble in water. It has a molecular weight of 101.1 g/mol and a melting point of 334°C. It is a strong oxidizing agent and can support combustion. Potassium nitrate is a source of nitrate and potassium ions, which are essential for plant growth.
Uses of Potassium Nitrate
1. Fertilizer: Potassium nitrate is a widely used source of nitrogen and potassium for plant nutrition. It is an important component of many fertilizers due to its high solubility and quick uptake by plants.
2. Food Preservation: It is used in the curing of meats such as bacon and salami to prevent the growth of harmful bacteria.
3. Pyrotechnics: Potassium nitrate is a key ingredient in the production of fireworks and smoke bombs due to its oxidizing properties.
4. Solid Rocket Propellant: It is used as an oxidizer in solid rocket propellants due to its ability to release oxygen during combustion.
Significance of Potassium Nitrate
Potassium nitrate plays a significant role in agriculture, food preservation, and the production of pyrotechnic materials. Its use as a fertilizer helps to enhance crop yields and promote plant growth. Additionally, its role in food preservation ensures the safety and longevity of various food products. In the field of pyrotechnics, it contributes to the vibrant colors and effects seen in fireworks displays.
Study Guide for Potassium Nitrate
1. What is the chemical formula of potassium nitrate?
The chemical formula of potassium nitrate is KNO3.
2. What are the properties of potassium nitrate?
Potassium nitrate is a colorless, odorless crystalline solid with a molecular weight of 101.1 g/mol and a melting point of 334°C. It is soluble in water and acts as a strong oxidizing agent.
3. Name three uses of potassium nitrate.
Three uses of potassium nitrate are as a fertilizer, in food preservation, and in pyrotechnics.
4. Why is potassium nitrate significant?
Potassium nitrate is significant due to its essential role in agriculture, food preservation, and the production of pyrotechnic materials. It enhances plant growth, ensures food safety, and contributes to the vibrant effects of fireworks.
.◂Science Worksheets and Study Guides Third Grade. Changes on earth
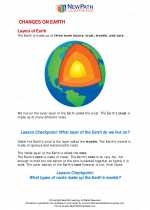
 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
