Oxidation
Oxidation is a chemical process in which a substance loses electrons. This can occur through a variety of chemical reactions, including the combination of a substance with oxygen, the removal of hydrogen atoms, or the loss of electrons to another substance. The substance that undergoes oxidation is referred to as the "reducing agent" because it causes the reduction (loss) of another substance.
Key Concepts
- Electron Loss: In oxidation, a substance loses electrons, which results in an increase in its oxidation state.
- Oxidizing Agent: The substance that causes another substance to undergo oxidation is known as the oxidizing agent.
- Redox Reactions: Oxidation-reduction (redox) reactions involve both oxidation and reduction processes.
- Oxidation State: The oxidation state of an element indicates the number of electrons it has gained or lost.
Examples of Oxidation
Oxidation is prevalent in a wide range of natural and industrial processes. Some common examples include:
- The rusting of iron, which occurs as a result of the reaction of iron with oxygen in the presence of moisture.
- The burning of fuels, such as the combustion of gasoline in a car engine, involves oxidation reactions.
- The metabolism of food in living organisms involves oxidation of nutrients to release energy.
Study Guide
To understand oxidation thoroughly, it is important to focus on the following aspects:
- Learn the concept of electron loss and its relationship to changes in oxidation state.
- Identify common oxidizing agents and their roles in causing oxidation reactions.
- Study redox reactions and how they involve both oxidation and reduction processes.
- Practice determining the oxidation states of elements in chemical compounds and reactions.
- Explore real-life examples of oxidation and its significance in various natural and industrial processes.
Understanding oxidation is crucial for comprehending a wide range of chemical reactions and processes. By mastering this concept, you will gain insight into the behavior of substances and the transformations they undergo in different environments.
.◂Science Worksheets and Study Guides Fourth Grade. Food webs/food chains

 Activity Lesson
Activity Lesson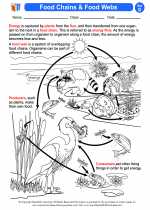
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
