Sublimation
Sublimation is the process in which a substance transitions directly from a solid to a gas without passing through the liquid state.
Explanation:
Sublimation occurs when the atmospheric pressure is lower than the vapor pressure of the solid substance. In this case, the solid absorbs heat and transforms into vapor without melting into a liquid first.
Examples of Sublimation:
- Carbon dioxide (dry ice) sublimes at room temperature and pressure, transitioning from a solid to a gas.
- Iodine also undergoes sublimation, turning directly from a solid to a purple vapor.
Study Guide:
When studying sublimation, consider the following key points:
- Understand the concept of sublimation as the direct transition from a solid to a gas.
- Learn about the conditions necessary for sublimation to occur, including the relationship between vapor pressure and atmospheric pressure.
- Memorize examples of substances that undergo sublimation in the natural world and their applications.
- Practice identifying sublimation in everyday scenarios, such as the behavior of dry ice.
◂Science Worksheets and Study Guides Sixth Grade. Cell Transport
Worksheet/Answer key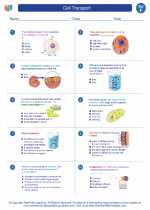 Cell Transport
Cell Transport  Worksheet/Answer key
Worksheet/Answer key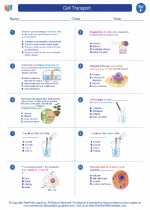 Cell Transport
Cell Transport  Vocabulary/Answer key
Vocabulary/Answer key Cell Transport
Cell Transport  Vocabulary/Answer key
Vocabulary/Answer key Cell Transport
Cell Transport 

 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key

The resources above cover the following skills:
Reading Standards for Literacy in Science and Technical Subjects
Craft and Structure
Determine the meaning of symbols, key terms, and other domain-specific words and phrases as they are used in a specific scientific or technical context relevant to grades 6-8 texts and topics.