Charles's Law
Charles's Law is a fundamental principle in the field of thermodynamics and gas laws. It describes how gases tend to expand when heated. The law is named after the French scientist Jacques Charles, who first formulated it in the 18th century.
Statement of Charles's Law
Charles's Law can be stated as follows: At constant pressure, the volume of a given mass of gas is directly proportional to its absolute temperature.
Mathematical Representation
The mathematical representation of Charles's Law is given by the equation:
V1 / T1 = V2 / T2
Where:
- V1 = Initial volume of the gas
- T1 = Initial temperature (in Kelvin)
- V2 = Final volume of the gas
- T2 = Final temperature (in Kelvin)
Key Concepts
Some key concepts related to Charles's Law include:
- Absolute temperature: Temperature must be expressed in Kelvin (K) to use Charles's Law. The Kelvin scale starts at absolute zero, where molecular motion theoretically ceases.
- Direct proportionality: This means that as the temperature of a gas increases, its volume also increases, and vice versa, as long as pressure is held constant.
- Constant pressure: Charles's Law applies only when the pressure on the gas remains the same throughout the process.
Applications
Charles's Law has numerous practical applications in various fields, including:
- Hot air balloons: The principle behind hot air balloons is based on Charles's Law, as heating the air inside the balloon causes it to expand and become less dense than the surrounding air.
- Thermometers: Many types of thermometers, such as gas thermometers, work on the principles described by Charles's Law.
- Cryogenics: Understanding the behavior of gases at different temperatures is crucial in the field of cryogenics, where extremely low temperatures are involved.
Study Guide
When studying Charles's Law, it's important to focus on the following key points:
- Understand the relationship between temperature and volume of a gas at constant pressure.
- Be able to convert temperatures to the Kelvin scale.
- Practice using the mathematical equation V1 / T1 = V2 / T2 to solve problems related to changes in volume and temperature of gases.
- Explore real-world examples and applications of Charles's Law to gain a deeper understanding of its significance.
By mastering Charles's Law, you'll gain valuable insights into the behavior of gases and be better equipped to understand various phenomena in the natural world as well as practical applications in technology and engineering.
.◂Science Worksheets and Study Guides Sixth Grade. Electricity

 Activity Lesson
Activity Lesson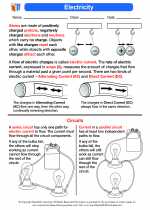
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
