Atomic Number
The atomic number of an element is the number of protons found in the nucleus of an atom. It is a unique identifier for each element and is used to arrange elements in the periodic table.
Key Points:
- The atomic number is represented by the symbol "Z".
- Elements with the same atomic number belong to the same chemical element.
- The atomic number determines the identity of an element and its chemical properties.
Importance:
Understanding the concept of atomic number is crucial in chemistry and physics. It helps in identifying elements, predicting their chemical behavior, and organizing them in the periodic table.
Study Tips:
- Memorize the atomic numbers of common elements.
- Practice using the periodic table to find the atomic number of different elements.
- Understand the relationship between atomic number and an element's position in the periodic table.
Example:
The atomic number of carbon is 6, which means it has 6 protons in its nucleus.
.◂Science Worksheets and Study Guides Sixth Grade. Magnetism
Study Guide Magnetism
Magnetism  Activity Lesson
Activity Lesson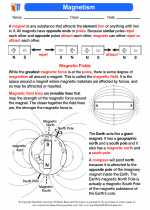 Magnetism
Magnetism  Worksheet/Answer key
Worksheet/Answer key Magnetism
Magnetism  Worksheet/Answer key
Worksheet/Answer key Magnetism
Magnetism  Worksheet/Answer key
Worksheet/Answer key Magnetism
Magnetism  Worksheet/Answer key
Worksheet/Answer key Magnetism
Magnetism  Vocabulary/Answer key
Vocabulary/Answer key Magnetism
Magnetism 

 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key

The resources above cover the following skills:
EARTH AND SPACE SCIENCE
Earth’s Systems
Develop and use models of Earth’s interior composition to illustrate the resulting magnetic field (e.g., magnetic poles) and to explain its measureable effects (e.g., protection from cosmic radiation).