Catalysts
A catalyst is a substance that speeds up a chemical reaction without being consumed in the reaction itself. In other words, it lowers the activation energy required for the reaction to occur, allowing it to happen more quickly.
Types of Catalysts
There are two main types of catalysts: homogeneous and heterogeneous catalysts. Homogeneous catalysts are in the same phase as the reactants, while heterogeneous catalysts are in a different phase.
How Catalysts Work
Catalysts work by providing an alternative pathway for the reaction to occur, which requires less energy. They achieve this by stabilizing the transition state of the reaction, making it easier for the reactants to form the products.
Examples of Catalysts
Common examples of catalysts include enzymes in biological systems, transition metals in industrial processes, and platinum in catalytic converters in cars.
Importance of Catalysts
Catalysts are crucial in many industrial processes as they allow for more efficient production of desired products. They also play a vital role in biological processes, allowing for the regulation of various biochemical reactions in living organisms.
Study Guide
- Define a catalyst and explain its role in chemical reactions.
- Describe the two main types of catalysts and provide examples of each.
- Explain how catalysts work to speed up chemical reactions.
- Give examples of catalysts used in industrial and biological processes.
- Discuss the importance of catalysts in chemical and biological systems.
◂Science Worksheets and Study Guides Sixth Grade. Magnetism

 Activity Lesson
Activity Lesson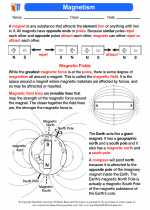
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
