What is Denitrification?
Denitrification is a microbial process that converts nitrates into nitrogen gas or nitrous oxide. It occurs in anaerobic environments such as waterlogged soils, wetlands, and sediments.Importance of Denitrification
Denitrification is important for the nitrogen cycle as it helps to return nitrogen gas to the atmosphere, balancing the nitrogen levels in the environment. It also prevents the accumulation of nitrates, which can be harmful to the environment in high concentrations.Steps of Denitrification
1. Nitrate Reduction: In the first step, nitrate (NO3-) is reduced to nitrite (NO2-) by denitrifying bacteria.2. Nitrite Reduction: Nitrite is further reduced to nitric oxide (NO) and then to nitrous oxide (N2O).3. Nitrous Oxide Reduction: Finally, nitrous oxide is further reduced to nitrogen gas (N2) by denitrifying bacteria, completing the process of denitrification.Factors Affecting Denitrification
- Temperature: Denitrification rates increase with higher temperatures. - pH: The process is optimal at a neutral pH (around 7). - Availability of Carbon: Denitrifying bacteria require carbon sources for energy to carry out denitrification.Environmental Impact
.◂Science Worksheets and Study Guides Sixth Grade. Magnetism
Study Guide Magnetism
Magnetism  Activity Lesson
Activity Lesson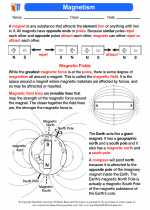 Magnetism
Magnetism  Worksheet/Answer key
Worksheet/Answer key Magnetism
Magnetism  Worksheet/Answer key
Worksheet/Answer key Magnetism
Magnetism  Worksheet/Answer key
Worksheet/Answer key Magnetism
Magnetism  Worksheet/Answer key
Worksheet/Answer key Magnetism
Magnetism  Vocabulary/Answer key
Vocabulary/Answer key Magnetism
Magnetism 

 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key

The resources above cover the following skills:
EARTH AND SPACE SCIENCE
Earth’s Systems
Develop and use models of Earth’s interior composition to illustrate the resulting magnetic field (e.g., magnetic poles) and to explain its measureable effects (e.g., protection from cosmic radiation).