Electrostatic Force
Electrostatic force is the force of attraction or repulsion between two charged objects. This force is caused by the interaction of charged particles, such as electrons and protons.
Key Concepts
- Charge: Objects become charged when they gain or lose electrons, resulting in a net positive or negative charge.
- Coulomb's Law: This law describes the force between two point charges and is given by the equation: F = k * (|q1 * q2|) / r^2, where F is the force, k is the electrostatic constant, q1 and q2 are the magnitudes of the charges, and r is the distance between the charges.
- Types of Forces: Electrostatic force can be attractive (opposite charges) or repulsive (like charges).
- Electric Fields: Charged objects create electric fields around them, which exert forces on other charged objects placed in the field.
Examples of Electrostatic Force
Some examples of electrostatic force in everyday life include:
- Attraction between a balloon and a wall after being rubbed on hair
- Repulsion between two negatively charged balloons
- Attraction between a positively charged balloon and a negatively charged balloon
Study Guide
When studying electrostatic force, make sure to understand the following key points:
- What causes an object to become charged?
- How does the distance between charges affect the strength of the electrostatic force?
- What is Coulomb's Law and how is it used to calculate the electrostatic force?
- What are the similarities and differences between gravitational force and electrostatic force?
- How do electric fields relate to electrostatic force?
Understanding these concepts will help you grasp the fundamentals of electrostatic force and its applications in various phenomena.
.◂Science Worksheets and Study Guides Sixth Grade. Magnetism
Study Guide Magnetism
Magnetism  Activity Lesson
Activity Lesson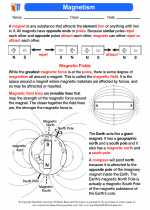 Magnetism
Magnetism  Worksheet/Answer key
Worksheet/Answer key Magnetism
Magnetism  Worksheet/Answer key
Worksheet/Answer key Magnetism
Magnetism  Worksheet/Answer key
Worksheet/Answer key Magnetism
Magnetism  Worksheet/Answer key
Worksheet/Answer key Magnetism
Magnetism  Vocabulary/Answer key
Vocabulary/Answer key Magnetism
Magnetism 

 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key

The resources above cover the following skills:
EARTH AND SPACE SCIENCE
Earth’s Systems
Develop and use models of Earth’s interior composition to illustrate the resulting magnetic field (e.g., magnetic poles) and to explain its measureable effects (e.g., protection from cosmic radiation).