Freezing Point
The freezing point of a substance is the temperature at which it changes from a liquid to a solid state. It is the temperature at which the vapor pressure of the solid and the liquid phases are equal and the substances exist in equilibrium. The freezing point is a characteristic property of a substance and can be used to identify and purify it.
Factors Affecting Freezing Point
The freezing point of a substance can be affected by various factors, including:
- Pressure: Generally, an increase in pressure leads to a decrease in the freezing point of a substance, and a decrease in pressure leads to an increase in the freezing point.
- Impurities: The presence of impurities in a substance can lower its freezing point. This is known as freezing point depression.
- Molecular Size and Structure: The size and structure of molecules can also affect the freezing point of a substance.
Study Guide
To understand the concept of freezing point, it is important to:
- Learn about the molecular structure and intermolecular forces of different substances.
- Understand the concept of equilibrium between the solid and liquid phases of a substance at its freezing point.
- Explore the effects of impurities and pressure on the freezing point of a substance.
- Practice solving problems related to freezing point depression and elevation.
Additional resources such as textbooks, online tutorials, and hands-on experiments can also aid in understanding the concept of freezing point.
.◂Science Worksheets and Study Guides Sixth Grade. Magnetism
Study Guide Magnetism
Magnetism  Activity Lesson
Activity Lesson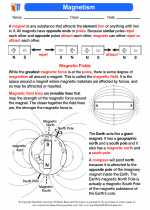 Magnetism
Magnetism  Worksheet/Answer key
Worksheet/Answer key Magnetism
Magnetism  Worksheet/Answer key
Worksheet/Answer key Magnetism
Magnetism  Worksheet/Answer key
Worksheet/Answer key Magnetism
Magnetism  Worksheet/Answer key
Worksheet/Answer key Magnetism
Magnetism  Vocabulary/Answer key
Vocabulary/Answer key Magnetism
Magnetism 

 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key

The resources above cover the following skills:
EARTH AND SPACE SCIENCE
Earth’s Systems
Develop and use models of Earth’s interior composition to illustrate the resulting magnetic field (e.g., magnetic poles) and to explain its measureable effects (e.g., protection from cosmic radiation).