Water
Water is a compound made up of two hydrogen atoms and one oxygen atom, represented by the chemical formula H2O. It is essential for all forms of life and plays a crucial role in many natural processes.
Properties of Water
- Universal Solvent: Water is known as the universal solvent because it has the ability to dissolve a wide range of substances.
- High Surface Tension: The cohesive forces between water molecules create a high surface tension, allowing some insects and small animals to walk on water.
- High Heat Capacity: Water has a high heat capacity, which means it can absorb and retain a large amount of heat without a significant increase in temperature.
- Expansion upon Freezing: Unlike most substances, water expands when it freezes, which is why ice floats on water.
States of Water
Water can exist in three states: solid, liquid, and gas.
- Solid (Ice): At temperatures below 0°C, water freezes and forms ice. Ice has a lower density than liquid water.
- Liquid: Water is most commonly found in its liquid state, which is essential for life as we know it.
- Gas (Water Vapor): When water is heated to its boiling point, it turns into water vapor, which is the gaseous form of water.
Importance of Water
Water is vital for various biological and environmental processes, including:
- Hydration: It is essential for the proper functioning of the human body and other organisms.
- Photosynthesis: Water is a key component in the process of photosynthesis, which is how plants produce their own food.
- Erosion and Weathering: Water plays a significant role in shaping the Earth's surface through erosion and weathering processes.
- Climate Regulation: Water has a significant impact on regulating the Earth's climate through processes such as evaporation and precipitation.
Study Guide
Here are some key points to remember when studying the topic of water:
- Understand the chemical structure of water and its unique properties.
- Learn about the different states of water and the processes involved in phase transitions.
- Explore the various roles of water in biological and environmental processes.
- Practice identifying real-life examples of water's significance in everyday life and the natural world.
Remember to stay hydrated and appreciate the incredible properties of water!
[Water] Related Worksheets and Study Guides:
.◂Science Worksheets and Study Guides Sixth Grade. Magnetism
Study Guide Magnetism
Magnetism  Activity Lesson
Activity Lesson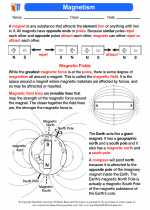 Magnetism
Magnetism  Worksheet/Answer key
Worksheet/Answer key Magnetism
Magnetism  Worksheet/Answer key
Worksheet/Answer key Magnetism
Magnetism  Worksheet/Answer key
Worksheet/Answer key Magnetism
Magnetism  Worksheet/Answer key
Worksheet/Answer key Magnetism
Magnetism  Vocabulary/Answer key
Vocabulary/Answer key Magnetism
Magnetism 

 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key

The resources above cover the following skills:
EARTH AND SPACE SCIENCE
Earth’s Systems
Develop and use models of Earth’s interior composition to illustrate the resulting magnetic field (e.g., magnetic poles) and to explain its measureable effects (e.g., protection from cosmic radiation).