Bases in Chemistry
In chemistry, a base is a substance that can accept hydrogen ions (H+) or donate hydroxide ions (OH-) in a chemical reaction. Bases are the opposite of acids, which can donate hydrogen ions. When a base is dissolved in water, it forms hydroxide ions, which can react with acids to form water and a salt.
Characteristics of Bases
- Taste: Bases often have a bitter taste.
- Touch: Some bases feel slippery or soapy to the touch.
- Reaction with Indicators: Bases turn red litmus paper blue and can change the color of other indicators.
- Electrical Conductivity: Bases can conduct electricity when dissolved in water.
Examples of Common Bases
- Sodium Hydroxide (NaOH): Found in drain cleaners and used in the production of soaps and detergents.
- Calcium Hydroxide (Ca(OH)2): Also known as slaked lime, used in construction and in the production of cement.
- Ammonia (NH3): Used in household cleaning products and as a fertilizer.
Importance of Bases
Bases play a crucial role in various industrial processes, including the production of soaps, detergents, and fertilizers. They are also used in the treatment of acidic soils and in the manufacturing of various chemicals.
Study Guide
When studying bases, it's important to understand the following key concepts:
- The definition of a base and its role in chemical reactions.
- The characteristics of bases, including taste, touch, and their reactions with indicators.
- Examples of common bases and their uses in everyday life and industry.
- The importance of bases in various industrial processes and applications.
Additionally, practice identifying bases in chemical equations and understanding their behavior in different reaction scenarios.
Understanding the properties and applications of bases is essential for a comprehensive understanding of chemistry and its practical applications in various fields.
.◂Science Worksheets and Study Guides Sixth Grade. Plant Processes

 Activity Lesson
Activity Lesson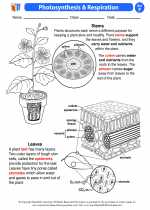
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
