Properties of Nitrogen
- Atomic number: 7
- Atomic symbol: N
- Atomic weight: 14.00674
- Physical state at room temperature: Gas
- Color: Colorless
- Odor: Odorless
Forms of Nitrogen
Nitrogen can exist in various forms, including:
- Nitrogen gas (N2): The most abundant form of nitrogen in the atmosphere.
- Ammonia (NH3): A compound of nitrogen and hydrogen, commonly used in fertilizers.
- Nitrites and Nitrates: Forms of nitrogen found in soil and water, important for plant nutrition.
- Amino acids and proteins: Organic compounds that contain nitrogen and are essential for life.
Uses of Nitrogen
Nitrogen has numerous industrial, agricultural, and biological applications, including:
- Manufacturing of Ammonia: Used in fertilizers and various chemical processes.
- Food Storage: Nitrogen gas is used to preserve food by preventing spoilage and oxidation.
- Coolant: Liquid nitrogen is used as a coolant in various industrial processes and scientific research.
- Purging and Inerting: Nitrogen is used to displace air and create inert atmospheres in manufacturing and storage facilities.
Environmental Impact
While nitrogen is essential for plant growth, excessive use of nitrogen-based fertilizers can lead to environmental issues such as water pollution and ecosystem disruption. This can result from the leaching of nitrates into groundwater and the release of nitrogen compounds into the atmosphere, contributing to air pollution and climate change.
Study Guide
When studying nitrogen, it's important to focus on its properties, forms, uses, and environmental impact. Consider the following study topics:
.◂Science Worksheets and Study Guides Sixth Grade. Plant Processes
Study Guide Plant Processes
Plant Processes  Activity Lesson
Activity Lesson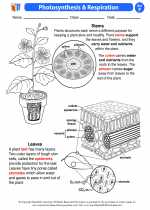 Photosynthesis & Respiration
Photosynthesis & Respiration  Worksheet/Answer key
Worksheet/Answer key Plant Processes
Plant Processes  Worksheet/Answer key
Worksheet/Answer key Plant Processes
Plant Processes  Worksheet/Answer key
Worksheet/Answer key Plant Processes
Plant Processes  Worksheet/Answer key
Worksheet/Answer key Plant Processes
Plant Processes  Vocabulary/Answer key
Vocabulary/Answer key Plant Processes
Plant Processes  Vocabulary/Answer key
Vocabulary/Answer key Plant Processes
Plant Processes 

 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key

The resources above cover the following skills:
LIFE SCIENCE
From Molecules to Organisms: Structures and Processes
Students who demonstrate understanding can:
Construct a scientific explanation based on evidence for the role of photosynthesis in the cycling of matter and flow of energy into and out of organisms.