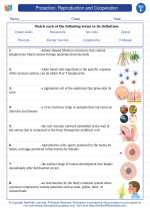
Spectroscopy
Spectroscopy is the study of the interaction between matter and electromagnetic radiation. It involves the analysis of how light is absorbed or emitted by different substances, providing valuable information about their composition, structure, and properties. Spectroscopy is widely used in various scientific fields such as chemistry, physics, astronomy, and environmental science.
Types of Spectroscopy
There are several types of spectroscopy, each with its own unique applications and techniques:
- Atomic Spectroscopy: This involves the study of the electromagnetic radiation absorbed and emitted by atoms. It includes techniques such as atomic absorption spectroscopy, atomic emission spectroscopy, and atomic fluorescence spectroscopy.
- Molecular Spectroscopy: This focuses on the interaction of electromagnetic radiation with molecules, providing information about their structure and bonding. Techniques include infrared spectroscopy, Raman spectroscopy, and nuclear magnetic resonance (NMR) spectroscopy.
- Ultraviolet-Visible (UV-Vis) Spectroscopy: This technique measures the absorption of ultraviolet and visible light by a substance, yielding information about its electronic transitions.
- Mass Spectrometry: This method analyzes the mass-to-charge ratio of ionized molecules, allowing for the determination of molecular weight and structural information.
- X-ray Spectroscopy: X-ray techniques are used to study the interaction of X-rays with matter, providing detailed information about the atomic and molecular structure of materials.
- Electron Spin Resonance (ESR) Spectroscopy: ESR spectroscopy is used to study materials with unpaired electrons, such as free radicals and transition metal ions, providing information about their electronic structure and magnetic properties.
Applications of Spectroscopy
Spectroscopy has a wide range of applications across different scientific disciplines:
- Chemical Analysis: Spectroscopic techniques are used to identify and quantify the components of complex mixtures, such as environmental samples, pharmaceuticals, and forensic evidence.
- Materials Characterization: Spectroscopy helps in understanding the composition, structure, and properties of materials, including polymers, semiconductors, and nanomaterials.
- Biochemical Studies: It is used to investigate the structure and function of biological molecules such as proteins, nucleic acids, and lipids, aiding in drug discovery and medical diagnostics.
- Astronomy: Spectroscopy is crucial in analyzing the light emitted or absorbed by celestial objects, providing insights into their chemical composition, temperature, and motion.
- Environmental Monitoring: It is used to analyze pollutants, greenhouse gases, and other environmental contaminants, aiding in environmental assessment and monitoring.
Study Guide
When studying spectroscopy, it is important to understand the basic principles and techniques of the different spectroscopic methods. Here are some key topics to focus on:
- Understanding the electromagnetic spectrum and the different regions of electromagnetic radiation used in spectroscopy.
- Learning about the interaction of light with matter, including absorption, emission, and scattering processes.
- Exploring the principles of different spectroscopic techniques, such as infrared spectroscopy, UV-Vis spectroscopy, and mass spectrometry.
- Understanding the applications of spectroscopy in various scientific fields and real-world scenarios.
- Practicing spectral interpretation and analysis, including understanding the information provided by different types of spectroscopic data.
By mastering these concepts and techniques, you will gain a comprehensive understanding of spectroscopy and its significance in scientific research and applications.
.◂Science Worksheets and Study Guides Sixth Grade. Protection, Reproduction and Cooperation
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key