Solids, Liquids, and Gases
In the study of matter, we often classify substances into three main states: solids, liquids, and gases. Each state has its own unique characteristics and properties, which we will explore below:
Solids
- Definition: Solids are substances that have a definite shape and volume. The particles in a solid are closely packed together and vibrate in place.
- Characteristics:
- Definite shape and volume
- Particles are tightly packed
- Strong intermolecular forces
- Do not flow easily
- Do not compress easily
Liquids
- Definition: Liquids are substances that have a definite volume but take the shape of their container. The particles in a liquid are close together but can move past each other.
- Characteristics:
- Definite volume, but no definite shape
- Particles can move past each other
- Moderate intermolecular forces
- Flow easily
- Do not compress easily
Gases
- Definition: Gases are substances that have neither a definite shape nor volume. The particles in a gas are far apart and move freely.
- Characteristics:
- No definite shape or volume
- Particles are far apart and move freely
- Weak intermolecular forces
- Flow easily
- High compressibility
Changes in States of Matter
Matter can change from one state to another through processes such as melting, freezing, evaporation, condensation, and sublimation. These changes are due to variations in temperature and pressure.
Study Guide
Now that you understand the properties of solids, liquids, and gases, here are some key points to remember for your study guide:
- What are the characteristics of solids, and how do they differ from liquids and gases?
- Explain the concept of intermolecular forces and its role in determining the properties of different states of matter.
- Describe the changes in states of matter and provide examples of each process.
- Discuss the relationship between temperature and pressure in causing transitions between states of matter.
Understanding the states of matter is essential in comprehending the behavior of substances in various conditions. Make sure to review these concepts and practice applying them to real-world examples to solidify your understanding.
.◂Science Worksheets and Study Guides Sixth Grade. Solids, liquids and gases

 Activity Lesson
Activity Lesson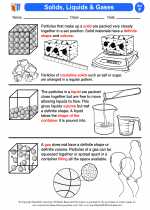
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
