Solids, liquids and gases -> gases
Gases
A gas is one of the four fundamental states of matter, along with solid, liquid, and plasma. Gases have properties that distinguish them from solids and liquids. These properties include their ability to expand to fill the space available to them, their compressibility, and the random motion of their particles. Here are some key concepts to understand about gases:
Gas Laws
Gas laws describe the behavior of gases under different conditions of temperature, pressure, and volume. The main gas laws include:
- Boyle's Law: Describes the inverse relationship between the pressure and volume of a gas, assuming constant temperature.
- Charles's Law: Describes the direct relationship between the volume and absolute temperature of a gas, assuming constant pressure.
- Gay-Lussac's Law: Describes the direct relationship between the pressure and absolute temperature of a gas, assuming constant volume.
- Combined Gas Law: Combines Boyle's, Charles's, and Gay-Lussac's laws into a single equation that relates pressure, volume, and temperature.
Gas Properties
Gases have several characteristic properties, including:
- Expansion: Gases do not have a fixed shape or volume and will expand to fill the container they are in.
- Compressibility: Gases can be compressed into a smaller volume under pressure.
- Diffusion: Gases spread out and mix with other gases due to the random motion of their particles.
- Pressure: The force exerted by gas particles on the walls of their container.
Gas Behavior
The behavior of gases can be described by the kinetic molecular theory, which states that gas particles are in constant, random motion and that their behavior can be explained by considering their motion and interactions with each other.
Study Guide
Here are some key points to focus on when studying gases:
- Understand the gas laws and how they relate to the behavior of gases.
- Learn about the properties of gases and how they differ from solids and liquids.
- Be able to explain the behavior of gases using the kinetic molecular theory.
- Practice solving problems involving the gas laws and gas properties.
By understanding these concepts and practicing problem-solving, you will be well-prepared to grasp the topic of gases.
.◂Science Worksheets and Study Guides Sixth Grade. Solids, liquids and gases

 Activity Lesson
Activity Lesson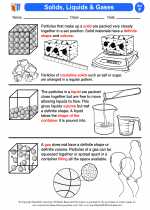
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
