Introduction to Chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a fundamental science that helps us understand the composition, structure, and properties of substances, as well as the changes they undergo. In this study guide, we will cover the basic concepts of chemistry that are essential for understanding the subject.
Atomic Structure
Atoms are the basic building blocks of matter. They consist of a nucleus, which contains protons and neutrons, surrounded by electrons. The number of protons in the nucleus determines the element's identity, while the number of neutrons can vary, resulting in different isotopes of the same element.
Chemical Bonding
Chemical bonding is the process by which atoms are held together to form compounds. There are different types of chemical bonds, including ionic, covalent, and metallic bonds. These bonds are formed through the sharing or transfer of electrons between atoms.
States of Matter
Matter exists in three primary states: solid, liquid, and gas. Changes in temperature and pressure can cause substances to undergo phase transitions, such as melting, freezing, vaporization, and condensation.
Chemical Reactions
Chemical reactions involve the rearrangement of atoms to form new substances. Reactants are the initial substances that undergo change, while products are the resulting substances. Chemical reactions can be described using chemical equations, which show the reactants and products involved.
Acids and Bases
Acids are substances that can donate protons, while bases are substances that can accept protons. The pH scale is used to measure the acidity or basicity of a solution, ranging from 0 (very acidic) to 14 (very basic).
Study Guide Questions
- What is an atom and what are its main components?
- Describe the difference between ionic and covalent bonding.
- Explain the three primary states of matter and give examples of each.
- How are chemical reactions represented using chemical equations?
- What is the pH scale and how is it used to classify substances?
Use these questions to guide your understanding of the key concepts in chemistry. Understanding these fundamental principles will provide a strong foundation for further exploration of this fascinating scientific field.
[Chemistry] Related Worksheets and Study Guides:
.◂Science Worksheets and Study Guides Seventh Grade. Cell Processes

 Activity Lesson
Activity Lesson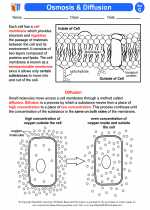
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
