What is Quantum Mechanics?
Quantum mechanics is a fundamental theory in physics that describes the behavior of matter and energy at atomic and subatomic scales. It provides a mathematical framework for understanding the wave-particle duality of particles, the uncertainty principle, and the probabilistic nature of physical phenomena at the quantum level.
Key Concepts in Quantum Mechanics
Wave-Particle Duality
The concept that particles such as electrons and photons exhibit both wave-like and particle-like properties. This means that they can behave as waves and particles depending on the conditions of the experiment.
Quantum Superposition
The principle that quantum systems can exist in multiple states simultaneously until they are measured or observed. This is often illustrated using the famous Schrödinger's cat thought experiment.
Quantization of Energy
The idea that energy is not continuous but exists in discrete, quantized amounts. This is exemplified in the energy levels of electrons in an atom and the emission or absorption of photons.
Uncertainty Principle
Proposed by Werner Heisenberg, this principle states that certain pairs of physical properties, such as position and momentum, cannot be simultaneously known to arbitrary precision. The more precisely one property is measured, the less precisely the other can be known.
Quantum Entanglement
The phenomenon in which two or more particles become correlated in such a way that the state of one particle cannot be described independently of the state of the others. This concept is essential in quantum computing and quantum cryptography.
Applications of Quantum Mechanics
Quantum mechanics has a wide range of applications in modern technology, including:
- Quantum computing
- Quantum cryptography
- Quantum teleportation
- Nanotechnology
- Quantum optics
Study Tips for Quantum Mechanics
Studying quantum mechanics can be challenging, but with the right approach, it can be an incredibly rewarding subject. Here are some tips to help you succeed:
- Understand the Mathematical Formalism: Quantum mechanics relies heavily on mathematical concepts such as complex numbers, linear algebra, and differential equations. Make sure to have a strong grasp of these mathematical tools.
- Visualize Quantum Concepts: Since quantum mechanics deals with phenomena at the atomic and subatomic scales, it can be helpful to develop visual models and analogies to understand abstract concepts.
- Practice Problem-Solving: Quantum mechanics is a problem-solving based subject. Practice solving problems and working through thought experiments to solidify your understanding of the concepts.
- Stay Updated: Quantum mechanics is a rapidly evolving field. Stay updated with the latest research and developments to gain a deeper understanding of the subject.
- Seek Help When Needed: If you're struggling with certain concepts, don't hesitate to seek help from your teacher, tutor, or online resources.
Conclusion
Quantum mechanics is a fascinating and essential theory that has revolutionized our understanding of the fundamental nature of reality. By studying and understanding the key concepts and applications of quantum mechanics, you can gain a deeper appreciation for the intricate workings of the universe and the potential for groundbreaking technological advancements.
.◂Science Worksheets and Study Guides Seventh Grade. Cell Processes

 Activity Lesson
Activity Lesson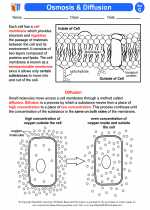
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
