Formation of Anions
Anions are formed through the process of electron gain. Non-metallic elements tend to gain electrons to achieve a stable outer electron configuration, typically with eight electrons in their outermost energy level. This results in the formation of negatively charged ions.Naming Convention for Anions
Anions are named by adding the suffix "-ide" to the root of the element's name. For example, when chlorine gains an electron to form an anion, it is named chloride (Cl-).Properties of Anions
- Anions are larger in size compared to their parent atoms due to the addition of extra electrons, which increases the electron-electron repulsion. - Anions are attracted to the positive terminal of a battery or external electric field due to their negative charge. - Anions can form ionic compounds with cations (positively charged ions) through electrostatic interactions.Study Guide for Anions
To study anions effectively, consider the following key points:- Understand the process of electron gain and the formation of anions.
- Learn the naming convention for anions and practice naming common anions formed by non-metallic elements.
- Explore the properties of anions, including their size, charge, and behavior in electric fields.
- Practice writing chemical formulas for ionic compounds formed by anions and cations.
- Engage in hands-on activities or simulations to visualize the behavior of anions in different chemical scenarios.
◂Science Worksheets and Study Guides Seventh Grade. Cell Reproduction
Study Guide Cell Reproduction
Cell Reproduction  Activity Lesson
Activity Lesson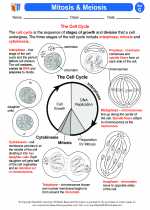 Mitosis & Meiosis
Mitosis & Meiosis  Worksheet/Answer key
Worksheet/Answer key Cell Reproduction
Cell Reproduction  Worksheet/Answer key
Worksheet/Answer key Cell Reproduction
Cell Reproduction  Worksheet/Answer key
Worksheet/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction 

 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key

The resources above cover the following skills:
LIFE SCIENCE
From Molecules to Organisms: Structures and Processes
Gather and synthesize information to explain how prokaryotic and eukaryotic cells differ in structure and function, including the methods of asexual and sexual reproduction.