Cell Reproduction -> corrosion
Corrosion
Corrosion is the process of deterioration of materials, usually metals, as a result of chemical or electrochemical reactions with their environment. It is a natural process that can be influenced by various factors such as moisture, oxygen, and other environmental conditions.
Causes of Corrosion
- Moisture: Water or humidity can accelerate the corrosion process.
- Oxygen: Presence of oxygen in the environment can cause oxidation of metals, leading to corrosion.
- Acids and Bases: Chemical substances with acidic or basic properties can corrode metals.
- Salt: Exposure to salt, such as in seawater or road salt, can accelerate corrosion.
Types of Corrosion
Corrosion can manifest in various forms, including:
- Uniform Corrosion: Generalized corrosion that occurs evenly across the surface of the metal.
- Galvanic Corrosion: Occurs when two different metals are in contact in the presence of an electrolyte, leading to accelerated corrosion of one of the metals.
- Pitting Corrosion: Localized corrosion that results in the formation of small pits on the metal surface.
- Crevice Corrosion: Occurs in confined spaces such as gaps and crevices where the access of oxygen and moisture is limited, leading to localized corrosion.
- Stress Corrosion Cracking: Occurs due to the combined influence of tensile stress and a corrosive environment, leading to cracking of the metal.
Prevention and Control
Corrosion can be prevented or controlled through various methods, including:
- Protective Coatings: Applying paints, varnishes, or specialized coatings to create a barrier between the metal and the environment.
- Galvanization: Coating the metal with a layer of zinc to provide sacrificial protection.
- Alloying: Mixing metals to form alloys that are more resistant to corrosion than pure metals.
- Cathodic Protection: Using sacrificial anodes or impressed current to protect the metal from corrosion.
- Proper Maintenance: Regular inspection, cleaning, and maintenance of metal surfaces to prevent corrosion.
Study Guide
Here are some key points to remember when studying corrosion:
- Define corrosion and explain its significance in everyday life.
- Identify the factors that contribute to corrosion.
- Describe the different types of corrosion and their characteristics.
- Explain the methods used to prevent and control corrosion.
- Discuss real-life examples of corrosion and its impact on various industries.
◂Science Worksheets and Study Guides Seventh Grade. Cell Reproduction
Study Guide Cell Reproduction
Cell Reproduction  Activity Lesson
Activity Lesson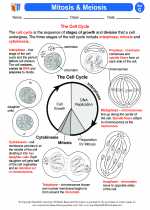 Mitosis & Meiosis
Mitosis & Meiosis  Worksheet/Answer key
Worksheet/Answer key Cell Reproduction
Cell Reproduction  Worksheet/Answer key
Worksheet/Answer key Cell Reproduction
Cell Reproduction  Worksheet/Answer key
Worksheet/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key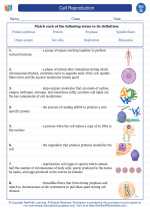 Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction 

 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key

The resources above cover the following skills:
LIFE SCIENCE
From Molecules to Organisms: Structures and Processes
Gather and synthesize information to explain how prokaryotic and eukaryotic cells differ in structure and function, including the methods of asexual and sexual reproduction.