Melting
Melting is the process by which a solid substance changes to a liquid state when heat is applied. This occurs when the thermal energy of the substance overcomes the attractive forces holding the particles in the solid together, causing them to move more freely and take on the characteristics of a liquid.
Key Concepts:
- Temperature: The increase in temperature provides the thermal energy necessary to break the bonds between the particles of a solid, causing it to melt.
- Heat Energy: Heat is the transfer of thermal energy from a hotter object to a cooler one. When heat is applied to a solid, its temperature rises, eventually reaching the melting point.
- Melting Point: The specific temperature at which a solid turns into a liquid is known as its melting point. Different substances have different melting points.
- Endothermic Process: Melting is an endothermic process, meaning it requires the absorption of heat energy to occur.
Study Guide:
Here are some key points to remember and study further:
- What is the definition of melting?
- What role does temperature play in the process of melting?
- Explain the concept of melting point and provide examples of substances with different melting points.
- How does the process of melting differ from the process of freezing?
- Discuss the concept of endothermic processes and explain why melting is considered an endothermic process.
Remember to review the properties of solids and liquids, as well as the behavior of particles in different states of matter to gain a deeper understanding of the process of melting.
Also, be sure to practice identifying and interpreting melting points on phase diagrams and understand the factors that can affect the melting point of a substance, such as pressure and impurities.
Finally, try conducting experiments to observe and measure the melting points of different substances to gain practical knowledge of the process.
Good luck with your studies! If you have any further questions, feel free to ask.
[Melting] Related Worksheets and Study Guides:
.◂Science Worksheets and Study Guides Seventh Grade. Cell Reproduction

 Activity Lesson
Activity Lesson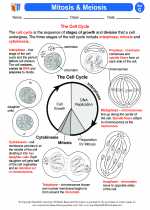
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
