What is Nitrogen?
Nitrogen is a chemical element with the symbol N and atomic number 7. It is a colorless, odorless, and tasteless gas at room temperature and makes up the majority of Earth's atmosphere.
Properties of Nitrogen:
- Atomic number: 7
- Atomic mass: 14.007 u
- Phase at room temperature: Gas
- Boiling point: -195.8°C
- Melting point: -210.0°C
- Density: 1.2506 g/L
- Electron configuration: 1s2 2s2 2p3
Uses of Nitrogen:
Nitrogen has various industrial and biological uses:
- As a component of ammonia, which is used in fertilizers
- In the production of nitric acid, which is used in explosives, plastics, and dyes
- In the food packaging industry to prevent spoilage and maintain product freshness
- In the production of electronic components and semiconductors
- As a coolant in certain applications due to its low temperature when in liquid form
Nitrogen Cycle:
Nitrogen undergoes various transformations in the environment through processes such as nitrogen fixation, nitrification, assimilation, ammonification, and denitrification. These processes are collectively known as the nitrogen cycle and are vital for the recycling and availability of nitrogen in ecosystems.
Study Tips:
To understand the topic of nitrogen better, consider the following study tips:
- Learn the different forms of nitrogen in the environment, such as nitrate, nitrite, and atmospheric nitrogen gas.
- Understand the role of nitrogen-fixing bacteria in converting atmospheric nitrogen into a form that plants can use.
- Explore the human impact on the nitrogen cycle, including the use of synthetic fertilizers and the effects of nitrogen pollution on the environment.
- Review the industrial applications of nitrogen and its importance in various fields such as agriculture, chemistry, and manufacturing.
- Practice solving problems related to calculating the percentage of nitrogen in compounds and understanding its chemical reactions.
◂Science Worksheets and Study Guides Seventh Grade. Cell Reproduction
Study Guide Cell Reproduction
Cell Reproduction  Activity Lesson
Activity Lesson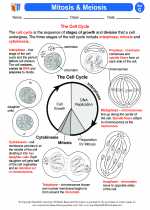 Mitosis & Meiosis
Mitosis & Meiosis  Worksheet/Answer key
Worksheet/Answer key Cell Reproduction
Cell Reproduction  Worksheet/Answer key
Worksheet/Answer key Cell Reproduction
Cell Reproduction  Worksheet/Answer key
Worksheet/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction 

 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key

The resources above cover the following skills:
LIFE SCIENCE
From Molecules to Organisms: Structures and Processes
Gather and synthesize information to explain how prokaryotic and eukaryotic cells differ in structure and function, including the methods of asexual and sexual reproduction.