Plasma
Plasma is the fourth state of matter, alongside solid, liquid, and gas. It is a state of matter that is not commonly found on Earth, but is abundant in the universe. Plasma is a gas in which a significant proportion of the particles are ionized. This means that the atoms in the gas have lost or gained electrons, resulting in the presence of positively and negatively charged particles. As a result, plasma displays unique properties that distinguish it from the other states of matter.
Characteristics of Plasma:
- Ionization: The presence of positively and negatively charged particles due to the loss or gain of electrons by atoms.
- Conductivity: Plasma can conduct electricity due to the movement of charged particles.
- Temperature: Plasma can reach extremely high temperatures, making it capable of producing light and heat.
- Magnetic properties: Plasma can be influenced by magnetic fields, and can also generate its own magnetic fields.
- State of the universe: Plasma is the most abundant state of matter in the universe, found in stars, nebulas, and other astronomical bodies.
Examples of Plasma:
Some common examples of plasma include:
- Stars: The Sun and other stars are composed of plasma.
- Lightning: The ionized air in a lightning bolt is an example of plasma.
- Neon signs: The colorful lights in neon signs are created using plasma.
- Auroras: The beautiful light displays in the polar regions are a result of interactions between solar winds and the Earth's atmosphere, creating plasma.
Study Guide:
Here are some key points to remember about plasma:
- What is plasma?
- How does plasma differ from the other states of matter?
- What are the characteristics of plasma?
- Provide examples of plasma in the universe and everyday life.
- Why is plasma important in the study of astrophysics?
- Explain the role of plasma in conducting electricity.
Understanding the properties and significance of plasma can provide insights into the behavior of matter in extreme conditions, as well as its role in the universe.
[Plasma] Related Worksheets and Study Guides:
.◂Science Worksheets and Study Guides Seventh Grade. Cell Reproduction
Study Guide Cell Reproduction
Cell Reproduction  Activity Lesson
Activity Lesson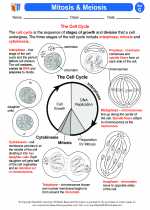 Mitosis & Meiosis
Mitosis & Meiosis  Worksheet/Answer key
Worksheet/Answer key Cell Reproduction
Cell Reproduction  Worksheet/Answer key
Worksheet/Answer key Cell Reproduction
Cell Reproduction  Worksheet/Answer key
Worksheet/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key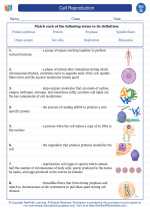 Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction 

 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key

The resources above cover the following skills:
LIFE SCIENCE
From Molecules to Organisms: Structures and Processes
Gather and synthesize information to explain how prokaryotic and eukaryotic cells differ in structure and function, including the methods of asexual and sexual reproduction.