Solid
A solid is one of the three main states of matter. Solids have a definite shape and volume, and the particles in a solid are tightly packed together in a regular pattern. This arrangement of particles allows solids to maintain their shape and resist flowing. Examples of solids include ice, wood, and metal.
Properties of Solids
Some key properties of solids include:
- Definite shape: Solids have a fixed shape and maintain their form unless acted upon by an external force.
- Definite volume: The volume of a solid remains constant, as the particles are closely packed together.
- High density: Solids are typically denser than liquids and gases due to the close packing of particles.
- Low compressibility: The particles in a solid are tightly packed, making it difficult to compress the solid.
- Strong intermolecular forces: The forces holding the particles together in a solid are strong, leading to the rigidity of the solid.
Types of Solids
Solids can be classified into different types based on the arrangement of particles:
- Crystalline solids: These solids have a regular, repeating pattern of particles, giving them distinct melting points and well-defined shapes. Examples include salt and diamond.
- Amorphous solids: In contrast to crystalline solids, amorphous solids do not have a regular pattern of particles. They tend to soften over a range of temperatures rather than having a specific melting point. Examples include glass and plastic.
Studying Solids
When studying solids, it's important to understand their properties, types, and behavior under different conditions. Here are some key points to focus on:
- Learn the difference between crystalline and amorphous solids, and be able to identify examples of each.
- Understand the concept of particles in a solid and how they are arranged.
- Explore the effects of temperature and pressure on solids, such as melting and boiling points.
- Examine the relationship between the structure of solids and their physical properties.
- Consider real-life applications of different types of solids, from construction materials to technological uses.
By grasping these fundamental concepts, you can gain a deeper understanding of the behavior and significance of solids in the world around us.
[Solid] Related Worksheets and Study Guides:
.◂Science Worksheets and Study Guides Seventh Grade. Cell Reproduction
Study Guide Cell Reproduction
Cell Reproduction  Activity Lesson
Activity Lesson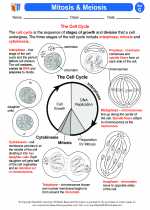 Mitosis & Meiosis
Mitosis & Meiosis  Worksheet/Answer key
Worksheet/Answer key Cell Reproduction
Cell Reproduction  Worksheet/Answer key
Worksheet/Answer key Cell Reproduction
Cell Reproduction  Worksheet/Answer key
Worksheet/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key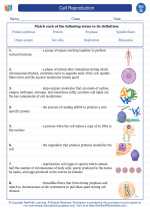 Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction 

 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key

The resources above cover the following skills:
LIFE SCIENCE
From Molecules to Organisms: Structures and Processes
Gather and synthesize information to explain how prokaryotic and eukaryotic cells differ in structure and function, including the methods of asexual and sexual reproduction.