Cell Reproduction -> solubility
Solubility
Solubility is the ability of a substance to dissolve in a solvent. The solvent can be a liquid, solid, or gas. The substance that is being dissolved is called the solute, and the solvent is the substance that dissolves the solute. The amount of solute that can be dissolved in a solvent at a specific temperature is known as the solubility of the substance.
Factors Affecting Solubility
The solubility of a substance can be affected by several factors:
- Temperature: In general, the solubility of most solid solutes increases as the temperature of the solvent increases. However, for some gases, solubility decreases as temperature increases.
- Pressure: The solubility of a gas in a liquid is directly proportional to the pressure of the gas above the liquid. This means that as the pressure of the gas increases, its solubility in the liquid also increases.
- Nature of the solute and solvent: The chemical nature of the solute and solvent can also affect solubility. For example, polar solutes tend to dissolve in polar solvents, while nonpolar solutes dissolve in nonpolar solvents.
Units of Solubility
The solubility of a substance is usually expressed in terms of the amount of solute that can be dissolved in a given amount of solvent. Common units of solubility include grams per 100 milliliters (g/100 mL) or grams per liter (g/L) for solids, and moles per liter (mol/L) for gases.
How to Determine Solubility
There are several methods for determining the solubility of a substance, including:
- Using a solubility chart or table that lists the solubility of different substances in various solvents at different temperatures.
- Conducting a solubility experiment in the laboratory, where the solute is added to a solvent and the amount of solute that dissolves is measured.
- Using mathematical models and equations to predict solubility based on the properties of the solute and solvent.
Applications of Solubility
Solubility has important applications in various fields, including:
- Chemistry: Solubility plays a crucial role in chemical reactions, as it determines the amount of reactants that can come into contact with each other in a solution.
- Pharmacy and medicine: The solubility of drugs in different solvents is important for formulating medications and determining their effectiveness.
- Environmental science: Understanding the solubility of pollutants in water is essential for evaluating their impact on the environment and developing remediation strategies.
Study Guide
Here are some key points to remember about solubility:
- Define solubility and distinguish between solute and solvent.
- Understand the factors that affect solubility, including temperature, pressure, and the nature of the solute and solvent.
- Be familiar with the units of solubility and how to express solubility in different units.
- Know the methods for determining solubility, such as using solubility charts, conducting experiments, and using mathematical models.
- Recognize the applications of solubility in different scientific and practical contexts.
Understanding solubility is important for various scientific disciplines and has practical implications in everyday life. By mastering the concept of solubility, you can gain a deeper understanding of chemical processes and their impact on the world around us.
[Solubility] Related Worksheets and Study Guides:
.◂Science Worksheets and Study Guides Seventh Grade. Cell Reproduction

 Activity Lesson
Activity Lesson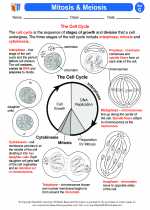
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
