Adsorption
Adsorption is the process by which molecules of a substance adhere to the surface of a solid or liquid material. This phenomenon occurs due to the attractive forces between the adsorbate (the substance being adsorbed) and the surface of the adsorbent (the material to which the adsorbate adheres).
Types of Adsorption
There are two main types of adsorption:
- Physical Adsorption (Physisorption): This type of adsorption involves weak van der Waals forces between the adsorbate and the adsorbent. It is typically reversible and occurs at the surface of the adsorbent.
- Chemical Adsorption (Chemisorption): Chemisorption involves the formation of chemical bonds between the adsorbate and the adsorbent. This type of adsorption is more specific and stronger than physical adsorption.
Factors Affecting Adsorption
Several factors can influence the process of adsorption:
- Surface Area: A larger surface area of the adsorbent allows for more adsorption sites and increases the potential for adsorption.
- Temperature: Generally, adsorption increases with a decrease in temperature.
- Pressure: Higher pressure can enhance adsorption, particularly for gases.
- Nature of Adsorbate and Adsorbent: The chemical and physical properties of the adsorbate and adsorbent play a significant role in adsorption.
Applications of Adsorption
Adsorption has various practical applications, including:
- Water purification
- Gas masks for protection against toxic gases
- Chromatography in chemistry
- Catalysis in chemical reactions
Study Guide for Adsorption
When studying adsorption, it is important to understand the following key points:
.◂Science Worksheets and Study Guides Seventh Grade. Earthquakes
Study Guide Earthquakes
Earthquakes  Activity Lesson
Activity Lesson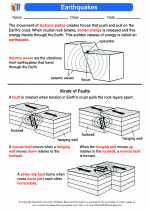 Earthquakes
Earthquakes  Worksheet/Answer key
Worksheet/Answer key Earthquakes
Earthquakes  Worksheet/Answer key
Worksheet/Answer key Earthquakes
Earthquakes  Worksheet/Answer key
Worksheet/Answer key Earthquakes
Earthquakes  Worksheet/Answer key
Worksheet/Answer key Earthquakes
Earthquakes  Worksheet/Answer key
Worksheet/Answer key Earthquakes
Earthquakes  Vocabulary/Answer key
Vocabulary/Answer key Earthquakes
Earthquakes  Vocabulary/Answer key
Vocabulary/Answer key Earthquakes
Earthquakes  Vocabulary/Answer key
Vocabulary/Answer key Earthquakes
Earthquakes  Vocabulary/Answer key
Vocabulary/Answer key Earthquakes
Earthquakes  Vocabulary/Answer key
Vocabulary/Answer key Earthquakes
Earthquakes  Vocabulary/Answer key
Vocabulary/Answer key Earthquakes
Earthquakes  Vocabulary/Answer key
Vocabulary/Answer key Earthquakes
Earthquakes 

 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
