Surface Energy
Surface energy is the energy required to increase the surface area of a liquid by a unit amount. It is a measure of the attraction between molecules at the surface of a liquid. The molecules at the surface have fewer neighboring molecules to attract to, so they have higher potential energy compared to the molecules in the bulk of the liquid.
Factors Affecting Surface Energy
- Molecular Attraction: The strength of attraction between molecules affects the surface energy. Stronger attraction results in higher surface energy.
- Temperature: Surface energy decreases with an increase in temperature, as the kinetic energy of molecules increases, reducing the strength of the intermolecular forces at the surface.
- Surface Area: Increasing the surface area of a liquid increases the surface energy.
Applications
Understanding surface energy is important in various fields, including materials science, nanotechnology, and chemistry. It affects phenomena such as wetting, adhesion, and the behavior of liquids in containers and on surfaces.
Study Guide
- Define surface energy and explain its significance in the behavior of liquids.
- Discuss the factors that affect surface energy and provide examples of each factor.
- Explain the relationship between temperature and surface energy, and how it impacts the behavior of liquids.
- Describe the applications of surface energy in real-world scenarios and provide specific examples.
- Compare and contrast the concepts of surface energy and surface tension, highlighting their similarities and differences.
◂Science Worksheets and Study Guides Seventh Grade. Earthquakes
Study Guide Earthquakes
Earthquakes  Activity Lesson
Activity Lesson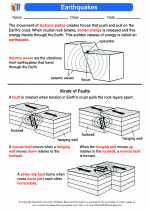 Earthquakes
Earthquakes  Worksheet/Answer key
Worksheet/Answer key Earthquakes
Earthquakes  Worksheet/Answer key
Worksheet/Answer key Earthquakes
Earthquakes  Worksheet/Answer key
Worksheet/Answer key Earthquakes
Earthquakes  Worksheet/Answer key
Worksheet/Answer key Earthquakes
Earthquakes  Worksheet/Answer key
Worksheet/Answer key Earthquakes
Earthquakes  Vocabulary/Answer key
Vocabulary/Answer key Earthquakes
Earthquakes  Vocabulary/Answer key
Vocabulary/Answer key Earthquakes
Earthquakes  Vocabulary/Answer key
Vocabulary/Answer key Earthquakes
Earthquakes  Vocabulary/Answer key
Vocabulary/Answer key Earthquakes
Earthquakes  Vocabulary/Answer key
Vocabulary/Answer key Earthquakes
Earthquakes  Vocabulary/Answer key
Vocabulary/Answer key Earthquakes
Earthquakes  Vocabulary/Answer key
Vocabulary/Answer key Earthquakes
Earthquakes 

 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
