Earthquakes -> water
Water
Water is a colorless, tasteless, and odorless liquid found on Earth. It is the most abundant substance on the planet and is essential for all forms of life.
Physical Properties of Water
- State: Water exists in three states: solid (ice), liquid (water), and gas (water vapor).
- Boiling Point: The boiling point of water is 100 degrees Celsius.
- Freezing Point: The freezing point of water is 0 degrees Celsius.
- Density: The density of water is 1 gram per cubic centimeter.
- Surface Tension: Water has a high surface tension, allowing some insects and small objects to float on its surface.
Chemical Properties of Water
- Chemical Formula: The chemical formula of water is H2O, indicating that each water molecule is composed of two hydrogen atoms and one oxygen atom.
- Polarity: Water is a polar molecule, meaning it has a slight positive charge on the hydrogen side and a slight negative charge on the oxygen side.
- Universal Solvent: Water is often referred to as the universal solvent due to its ability to dissolve a wide range of substances.
- Heat Capacity: Water has a high heat capacity, meaning it can absorb and release large amounts of heat with minimal temperature change.
Importance of Water
Water plays a crucial role in various biological, physical, and chemical processes. It is essential for hydration, supporting ecosystems, transportation of nutrients, and regulating body temperature.
Study Guide
Here are some key points to remember when studying the topic of water:
- Identify the physical states of water and their corresponding temperature points.
- Understand the chemical composition and polarity of water molecules.
- Explain the significance of water as a solvent and its role in biological systems.
- Discuss the importance of water for human health and environmental sustainability.
Remember to review the properties and functions of water thoroughly to gain a comprehensive understanding of this essential substance.
[Water] Related Worksheets and Study Guides:
.◂Science Worksheets and Study Guides Seventh Grade. Earthquakes
Study Guide Earthquakes
Earthquakes  Activity Lesson
Activity Lesson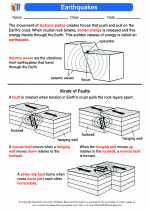 Earthquakes
Earthquakes  Worksheet/Answer key
Worksheet/Answer key Earthquakes
Earthquakes  Worksheet/Answer key
Worksheet/Answer key Earthquakes
Earthquakes  Worksheet/Answer key
Worksheet/Answer key Earthquakes
Earthquakes  Worksheet/Answer key
Worksheet/Answer key Earthquakes
Earthquakes  Worksheet/Answer key
Worksheet/Answer key Earthquakes
Earthquakes  Vocabulary/Answer key
Vocabulary/Answer key Earthquakes
Earthquakes  Vocabulary/Answer key
Vocabulary/Answer key Earthquakes
Earthquakes  Vocabulary/Answer key
Vocabulary/Answer key Earthquakes
Earthquakes  Vocabulary/Answer key
Vocabulary/Answer key Earthquakes
Earthquakes  Vocabulary/Answer key
Vocabulary/Answer key Earthquakes
Earthquakes  Vocabulary/Answer key
Vocabulary/Answer key Earthquakes
Earthquakes  Vocabulary/Answer key
Vocabulary/Answer key Earthquakes
Earthquakes 

 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
