Atomic Mass
Atomic mass is the mass of an atom of a chemical element, expressed in atomic mass units (amu). It is a fundamental property of the element and is a key factor in determining the element's chemical behavior.
Understanding Atomic Mass
The atomic mass of an element is determined by the total number of protons and neutrons in the nucleus of an atom. Since protons and neutrons have approximately the same mass, the atomic mass is often very close to the total number of protons and neutrons in the nucleus.
Calculating Atomic Mass
The atomic mass of an element is calculated by taking the weighted average of the isotopes of that element. Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. The atomic mass is calculated by multiplying the mass of each isotope by its natural abundance (as a decimal) and then adding the products together.
Study Guide for Atomic Mass
- Define atomic mass and explain its significance in determining the behavior of an element.
- Describe how the atomic mass is determined by the number of protons and neutrons in the nucleus of an atom.
- Explain the concept of isotopes and how they contribute to the calculation of atomic mass.
- Calculate the atomic mass of an element given the isotopic masses and natural abundances of its isotopes.
- Discuss the units in which atomic mass is expressed and its relationship to the unified atomic mass unit (amu).
- Compare and contrast atomic mass with atomic number, emphasizing their distinct roles in defining an element.
◂Science Worksheets and Study Guides Seventh Grade. Ecosystems, food chains and food webs

 Activity Lesson
Activity Lesson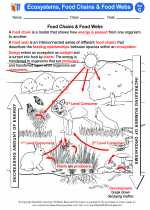
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
