Ecosystems, food chains and food webs -> boiling point
Boiling Point
The boiling point of a substance is the temperature at which the vapor pressure of the liquid equals the external pressure surrounding the liquid, and the liquid changes into a gas. It is a physical property that is unique to each substance and can be used to identify and characterize different materials.
Factors Affecting Boiling Point
- Intermolecular Forces: The strength of the forces between molecules affects the boiling point. Substances with stronger intermolecular forces require more energy to separate the molecules and thus have higher boiling points.
- Molecular Weight: Generally, substances with higher molecular weights have higher boiling points due to increased intermolecular forces.
- Pressure: Changes in external pressure can affect the boiling point. Higher pressures increase the boiling point, while lower pressures decrease it.
Measurement of Boiling Point
The boiling point of a substance is measured using a thermometer and a setup that allows the substance to be heated at a constant rate. The temperature at which the first bubble of vapor forms and escapes from the liquid is recorded as the boiling point, as this indicates that the vapor pressure equals the atmospheric pressure.
Real-World Applications
Boiling points are used in various industries and everyday life. For example, in the kitchen, knowing the boiling point of water helps in cooking food. In chemistry, it is used to purify substances through techniques such as distillation. Additionally, in the petroleum industry, knowledge of the boiling points of different hydrocarbons is crucial for refining processes.
Example Questions
- What is the boiling point of water at sea level?
- How does the boiling point of ethanol compare to that of methanol?
- Explain the effect of pressure on the boiling point of a substance.
.
◂Science Worksheets and Study Guides Seventh Grade. Ecosystems, food chains and food webs

 Activity Lesson
Activity Lesson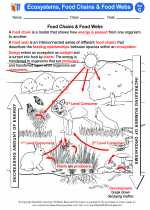
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
