What is Calcium Carbonate?
Calcium carbonate is a chemical compound with the formula CaCO3. It is a common substance found in rocks as the minerals calcite and aragonite and is the main component of pearls and the shells of marine organisms, snails, and eggs.
Properties of Calcium Carbonate
- Chemical Formula: CaCO3
- Appearance: White, odorless powder
- Solubility: Insoluble in water
- Uses: Calcium carbonate is used in the construction industry, in the production of cement, as a dietary supplement, in the manufacturing of paper, plastics, paints, and more.
Study Guide for Calcium Carbonate
Chemical Structure
Calcium carbonate is composed of calcium (Ca), carbon (C), and oxygen (O) atoms. It has a trigonal planar molecular geometry and is formed by the reaction of calcium ions (Ca2+) with carbonate ions (CO32-).
Formation in Nature
Calcium carbonate is often formed in nature through the reaction of calcium ions in water with carbonate ions, leading to the precipitation of calcium carbonate. This process is responsible for the formation of stalactites and stalagmites in caves.
Industrial Uses
Calcium carbonate has numerous industrial applications. It is used as a filler in the production of plastics, as a component of cement and mortar, in the manufacturing of glass, and in the production of paper. It serves as a dietary supplement to provide calcium, and is used as an antacid to neutralize stomach acid.
Chemical Reactions
Calcium carbonate undergoes decomposition when heated to high temperatures, producing calcium oxide (quicklime) and carbon dioxide gas. This reaction is used in the production of quicklime, an important industrial chemical.
Environmental Impact
Calcium carbonate plays a crucial role in the carbon cycle, as it is a major component of the shells of marine organisms and the exoskeletons of many organisms. Its dissolution in seawater also helps to regulate pH and carbon dioxide levels in the oceans.
Understanding the properties and uses of calcium carbonate is important in various fields including chemistry, geology, environmental science, and industrial applications.
.◂Science Worksheets and Study Guides Seventh Grade. Ecosystems, food chains and food webs

 Activity Lesson
Activity Lesson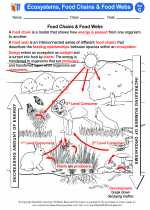
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
