Crystallization
Crystallization is the process of forming a solid, well-defined crystal structure from a liquid or gas. This process is commonly used in chemistry to purify substances or to produce crystals for various purposes.
How does crystallization occur?
Crystallization occurs when the particles in a solution, typically a liquid, come together to form a solid crystal lattice. This process is initiated by the cooling of the solution, leading to a decrease in the solubility of the solute. As a result, the excess solute particles start to come together and form solid crystals. The formation of crystals is highly dependent on the specific properties of the solute and solvent, as well as the rate of cooling or evaporation.
Applications of Crystallization
1. Purification of Substances: Crystallization is commonly used to purify substances by separating them from impurities in a solution. As the crystals form, impurities are left behind in the solution, leading to a purified substance in the crystal form.
2. Production of Crystals: Crystallization is used to produce crystals for various purposes such as in the manufacturing of pharmaceuticals, semiconductors, and other industrial processes.
Factors Affecting Crystallization
1. Solubility: The solubility of the solute in the solvent greatly affects the crystallization process. Lower solubility at lower temperatures promotes the formation of crystals.
2. Temperature: The rate of cooling or evaporation affects the size and quality of the crystals formed. Slow cooling or evaporation generally leads to larger and more well-defined crystals.
3. Stirring: Stirring the solution can help to promote the formation of crystals by ensuring even distribution of the solute in the solvent.
4. Impurities: The presence of impurities in the solution can hinder the crystallization process, leading to the formation of imperfect crystals.
Study Guide
Here are some key points to remember about crystallization:
- Crystallization is the process of forming a solid crystal structure from a liquid or gas.
- It is used for purification of substances and production of crystals for various applications.
- Factors affecting crystallization include solubility, temperature, stirring, and impurities.
- Understanding the properties of the solute and solvent is crucial for successful crystallization.
Practice Problems:
1. What are the key factors that affect the process of crystallization?
2. Explain how the process of crystallization is used to purify substances.
3. Why is it important to control the rate of cooling or evaporation during crystallization?
4. How does the presence of impurities impact the formation of crystals?
Further Reading:
1. Crystallization - Chemguide
2. Crystallization - ScienceDirect
.◂Science Worksheets and Study Guides Seventh Grade. Ecosystems, food chains and food webs

 Activity Lesson
Activity Lesson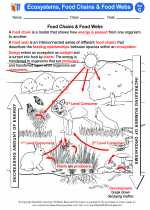
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
