Formation of Anions
When an atom gains one or more electrons, it becomes negatively charged and forms an anion. This process occurs when an atom interacts with other atoms, either through sharing or transferring of electrons.
Naming Anions
Anions are named by adding the suffix "-ide" to the root of the element's name. For example, when chlorine gains an electron, it forms the chloride anion.
Properties of Anions
- Anions are attracted to the anode in an electric field.
- They are often larger in size compared to the parent atom due to the addition of extra electrons.
- Anions are generally less reactive than cations, which are positively charged ions.
Study Guide
When studying anions, it's important to understand the following key points:
- How an anion is formed and the process of electron gain.
- The naming convention for anions.
- The properties and behavior of anions in chemical reactions and electric fields.
- How anions contribute to the formation of compounds and their role in various chemical processes.
It's also helpful to practice writing the chemical formulas for compounds containing anions and identifying the anion present in a given compound.
Understanding anions is fundamental to comprehending the behavior of ions in chemical reactions and the formation of ionic compounds. As you study, be sure to review the concepts and practice applying them in different contexts to solidify your understanding of anions and their significance in chemistry.
.◂Science Worksheets and Study Guides Eighth Grade. Circulation and immunity
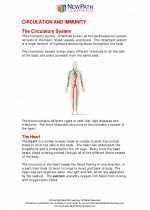
 Worksheet/Answer key
Worksheet/Answer key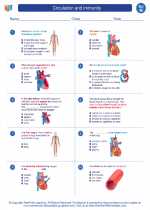
 Worksheet/Answer key
Worksheet/Answer key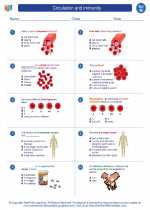
 Worksheet/Answer key
Worksheet/Answer key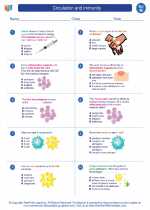
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
