Isotopes
An isotope is a variant of a particular chemical element which differs in neutron number, and consequently in atomic mass, but not in chemical properties. All isotopes of a given element have the same number of protons in each atom.
Key Points to Understand about Isotopes:
- Atomic Number: The number of protons in the nucleus of an atom determines the element's atomic number.
- Neutrons: Isotopes of an element have the same number of protons but different numbers of neutrons.
- Atomic Mass: The sum of protons and neutrons in the nucleus gives the atomic mass of an isotope.
- Nuclear Stability: Some isotopes are stable, while others are radioactive and decay over time.
Examples of Isotopes:
One common example of isotopes is carbon. Carbon-12, carbon-13, and carbon-14 are all isotopes of carbon, with 6 protons each but 6, 7, and 8 neutrons, respectively.
Study Guide Questions:
- What is an isotope?
- How do isotopes of an element differ from each other?
- Explain the concept of atomic mass in relation to isotopes.
- What is the difference between stable and radioactive isotopes?
- Provide examples of isotopes and their differences in neutron numbers.
Understanding isotopes is important in various scientific fields, including chemistry, physics, and geology. Mastery of this topic will provide a strong foundation for understanding atomic structure and the behavior of elements in nature and in the laboratory.
[Isotopes] Related Worksheets and Study Guides:
.◂Science Worksheets and Study Guides Kindergarten. All About Animals
Coloring Worksheet Birds
Birds  Coloring Worksheet
Coloring Worksheet Birds
Birds  Coloring Worksheet
Coloring Worksheet Bugs
Bugs  Coloring Worksheet
Coloring Worksheet Bugs
Bugs  Coloring Worksheet
Coloring Worksheet Butterfly life cycle
Butterfly life cycle  Coloring Worksheet
Coloring Worksheet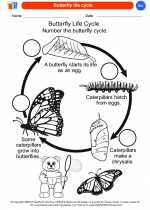 Butterfly life cycle
Butterfly life cycle  Coloring Worksheet
Coloring Worksheet Farm & Zoo
Farm & Zoo  Coloring Worksheet
Coloring Worksheet Farm & Zoo
Farm & Zoo  Coloring Worksheet
Coloring Worksheet Frog life cycle
Frog life cycle  Coloring Worksheet
Coloring Worksheet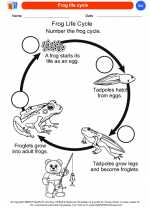 Frog life cycle
Frog life cycle  Coloring Worksheet
Coloring Worksheet Interdependence
Interdependence  Coloring Worksheet
Coloring Worksheet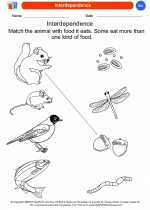 Interdependence
Interdependence  Coloring Worksheet
Coloring Worksheet Offspring
Offspring  Coloring Worksheet
Coloring Worksheet Offspring
Offspring  Coloring Worksheet
Coloring Worksheet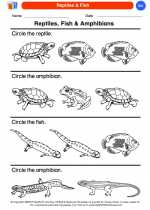 Reptiles & Fish
Reptiles & Fish  Coloring Worksheet
Coloring Worksheet Reptiles & Fish
Reptiles & Fish  Coloring Worksheet
Coloring Worksheet What animals need
What animals need  Coloring Worksheet
Coloring Worksheet What animals need
What animals need 

 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet

The resources above cover the following skills:
Science, Kindergarten, Adopted 2017 – The provisions of §§112.11-112.16 of this subchapter shall be implemented by school districts beginning with the 2018-2019 school year.
Knowledge and skills.
Organisms and environments. The student knows that organisms resemble their parents and have structures and processes that help them survive within their environments. The student is expected to:
sort plants and animals into groups based on physical characteristics such as color, size, body covering, or leaf shape
identify basic parts of plants and animals