Chemical Composition
Chemical composition refers to the identity and proportion of the elements that make up a substance. Understanding the chemical composition of a substance is important in various scientific fields, including chemistry, biology, and environmental science.
Elements and Atoms
Elements are the simplest form of substances and are made up of only one type of atom. Atoms are the basic building blocks of matter and are composed of protons, neutrons, and electrons. The number of protons in an atom determines the element it represents.
Chemical Formulas
Chemical formulas are used to represent the chemical composition of a substance. They provide information about the types and numbers of atoms in a compound. For example, the chemical formula for water is H2O, which indicates that a water molecule consists of two hydrogen atoms and one oxygen atom.
Molecular and Empirical Formulas
Molecular formulas show the actual number of each type of atom in a molecule, while empirical formulas show the simplest whole-number ratio of the atoms in a compound. For example, the molecular formula for glucose is C6H12O6, while the empirical formula is CH2O.
Study Guide
- Define chemical composition and explain its importance in scientific fields.
- Describe the structure of an atom and how it relates to the elements.
- Explain the difference between molecular and empirical formulas.
- Practice writing chemical formulas for simple compounds.
- Research the chemical composition of common substances such as water, salt, and sugar.
Understanding chemical composition is fundamental to understanding the properties and behavior of substances, and it forms the basis for many scientific concepts and applications.
.◂Science Worksheets and Study Guides Kindergarten. All About Plants

 Coloring Worksheet
Coloring Worksheet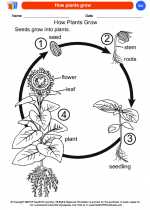
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet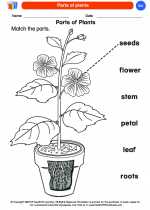
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet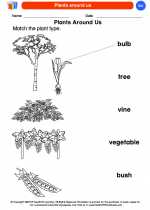
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet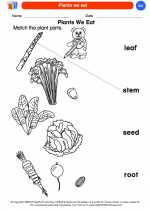
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
