All About Plants -> states of matter
States of Matter
Everything around us is made up of matter, and matter exists in different states. The three most common states of matter are solid, liquid, and gas.
Solid
A solid has a definite shape and volume. The particles in a solid are packed closely together and vibrate in place. They do not move around as freely as the particles in liquids or gases.
Liquid
A liquid has a definite volume, but it takes the shape of its container. The particles in a liquid are still close together, but they can move past each other. This allows liquids to flow and take the shape of their container.
Gas
A gas has neither a definite shape nor a definite volume. The particles in a gas are spread far apart and move freely. They fill the entire space available to them.
Changes in States of Matter
Matter can change from one state to another when energy is added or removed. For example, when a solid is heated, it can melt and become a liquid. When a liquid is heated further, it can vaporize and become a gas. This process is known as changing states of matter.
Study Guide
- What are the three most common states of matter?
- Describe the characteristics of a solid.
- Explain the properties of a liquid.
- How do the particles in a gas behave?
- What is the process called when matter changes from one state to another?
[States Of Matter] Related Worksheets and Study Guides:
.◂Science Worksheets and Study Guides Kindergarten. All About Plants

 Coloring Worksheet
Coloring Worksheet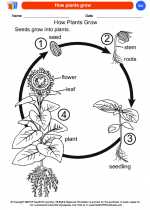
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet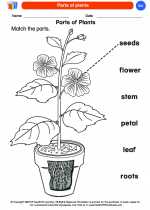
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet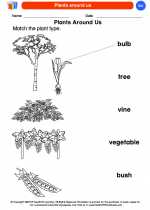
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet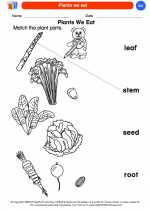
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
