Surface Tension
Surface tension is the tendency of the surface of a liquid to resist external force due to the cohesive nature of its molecules. It is what allows certain insects, like water striders, to walk on water and for water droplets to form a spherical shape.
Causes of Surface Tension
Surface tension is caused by the cohesive forces between the molecules of the liquid. These forces pull the molecules on the surface inward, creating a thin, strong "skin" on the surface of the liquid.
Measuring Surface Tension
Surface tension can be measured using a variety of methods, including the capillary rise method and the drop weight method. These methods help scientists and researchers understand the strength of the surface tension of different liquids.
Everyday Examples
Surface tension can be observed in everyday life. For example, when you overfill a glass of water, the water forms a dome-like shape above the rim before spilling over. This is due to the surface tension of the water.
Study Guide
- What is surface tension?
- What causes surface tension in liquids?
- How is surface tension measured?
- Provide examples of surface tension in everyday life.
Understanding surface tension is important in various scientific fields, including physics, chemistry, and biology. It also has practical applications in industries such as pharmaceuticals and material science.
[Surface Tension] Related Worksheets and Study Guides:
.◂Science Worksheets and Study Guides Kindergarten. All About Plants

 Coloring Worksheet
Coloring Worksheet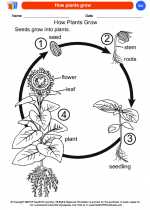
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet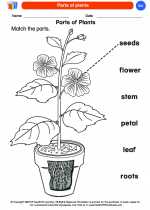
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet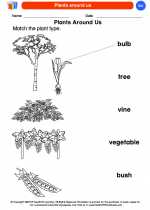
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet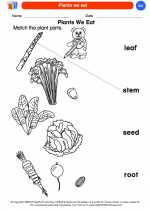
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
