Change
Change is all around us. In science, we learn about different types of changes that can occur, such as physical changes and chemical changes. Understanding these changes helps us make sense of the world around us.
Types of Changes
Physical Changes
Physical changes involve a change in appearance, shape, or state of matter without the substance becoming something new. For example, when water freezes into ice, it undergoes a physical change. The molecules in the water slow down and move closer together, but the substance is still water, just in a different form.
Chemical Changes
Chemical changes occur when substances react to form new substances with different properties. For example, when iron rusts, it undergoes a chemical change. The iron combines with oxygen in the air to form a new substance, iron oxide, which has different properties than the original iron.
Study Guide
- What is a physical change? Provide an example.
- What is a chemical change? Provide an example.
- What are some signs that a chemical change has occurred?
- Can a substance change back to its original form after undergoing a chemical change?
Answers
1. A physical change involves a change in appearance, shape, or state of matter without the substance becoming something new. An example of a physical change is when water evaporates into water vapor.
2. A chemical change occurs when substances react to form new substances with different properties. An example of a chemical change is when wood burns and turns into ash and smoke.
3. Some signs that a chemical change has occurred include the production of gas, a change in color, the release of energy in the form of heat or light, or the formation of a precipitate (a solid that forms from a chemical reaction in a liquid).
4. In some cases, a substance can change back to its original form after undergoing a chemical change, but not always. It depends on the specific chemical reaction and the conditions involved.
[Change] Related Worksheets and Study Guides:
.◂Science Worksheets and Study Guides Kindergarten. Weather

 Coloring Worksheet
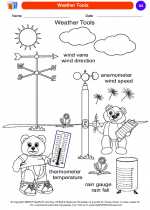
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet