Gas
Gas is one of the three common states of matter, alongside solids and liquids. Unlike solids and liquids, gases have no fixed shape or volume and will expand to fill the space available to them. The behavior of gases is governed by the principles of gas laws, including Boyle's law, Charles's law, and the ideal gas law.
Properties of Gases
- Expansion: Gases expand to fill the container they are placed in.
- Compressibility: Gases can be compressed into a smaller volume under pressure.
- Diffusion: Gases diffuse and mix with each other to form homogeneous mixtures.
- Low Density: Gases have low density compared to solids and liquids.
- No Fixed Shape or Volume: Gases do not have a fixed shape or volume.
Gas Laws
Gas laws describe the behavior of gases under different conditions. The three main gas laws are:
- Boyle's Law: This law states that at constant temperature, the volume of a given mass of gas is inversely proportional to its pressure.
- Charles's Law: This law states that at constant pressure, the volume of a given mass of gas is directly proportional to its absolute temperature.
- Ideal Gas Law: This law combines Boyle's and Charles's laws and introduces the concept of the ideal gas constant. It relates the pressure, volume, temperature, and number of moles of a gas.
Common Gases
Some common gases include:
Study Guide
When studying gases, it's important to understand the properties and behavior of gases, as well as the principles of gas laws. Here are some key points to focus on:
- Describe the properties of gases, including their ability to expand, compress, and diffuse.
- Explain the behavior of gases using the principles of gas laws, including Boyle's law, Charles's law, and the ideal gas law.
- Identify common gases and their uses in everyday life.
- Understand the factors that affect the behavior of gases, such as temperature, pressure, and volume.
By mastering these concepts, you will have a solid understanding of the topic of gases and their importance in the world around us.
.◂Science Worksheets and Study Guides Kindergarten. Weather
Coloring Worksheet Calendar
Calendar  Coloring Worksheet
Coloring Worksheet Calendar
Calendar  Coloring Worksheet
Coloring Worksheet Day and Night
Day and Night  Coloring Worksheet
Coloring Worksheet Day and Night
Day and Night  Coloring Worksheet
Coloring Worksheet Exploring Weather
Exploring Weather  Coloring Worksheet
Coloring Worksheet Exploring Weather
Exploring Weather  Coloring Worksheet
Coloring Worksheet Look at the Clouds
Look at the Clouds  Coloring Worksheet
Coloring Worksheet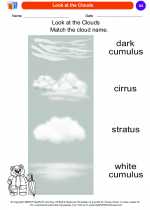 Look at the Clouds
Look at the Clouds  Coloring Worksheet
Coloring Worksheet Moon & Stars
Moon & Stars  Coloring Worksheet
Coloring Worksheet Moon & Stars
Moon & Stars  Coloring Worksheet
Coloring Worksheet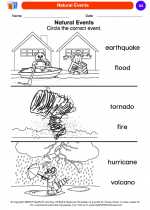 Natural Events
Natural Events  Coloring Worksheet
Coloring Worksheet Natural Events
Natural Events  Coloring Worksheet
Coloring Worksheet Sun and Shadows
Sun and Shadows  Coloring Worksheet
Coloring Worksheet Sun and Shadows
Sun and Shadows  Coloring Worksheet
Coloring Worksheet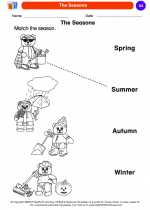 The Seasons
The Seasons  Coloring Worksheet
Coloring Worksheet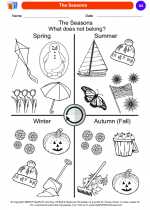 The Seasons
The Seasons  Coloring Worksheet
Coloring Worksheet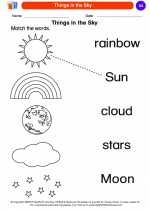 Things in the Sky
Things in the Sky  Coloring Worksheet
Coloring Worksheet Things in the Sky
Things in the Sky  Coloring Worksheet
Coloring Worksheet Weather Tools
Weather Tools  Coloring Worksheet
Coloring Worksheet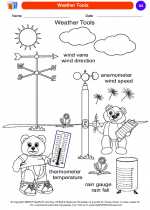 Weather Tools
Weather Tools 

 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet

The resources above cover the following skills:
EARTH AND SPACE SCIENCE (NGSS)
Earth’s Systems
Students who demonstrate understanding can:
Use and share observations of local weather conditions to describe patterns over time.