High Specific Heat
Specific heat is the amount of heat energy required to raise the temperature of a substance by a certain amount. When a substance has a high specific heat, it means that it can absorb a large amount of heat energy without its temperature increasing significantly.
Water is a great example of a substance with a high specific heat. This is because it takes a lot of energy to raise the temperature of water, and it can also release a lot of energy when it cools down.
Study Guide
- Definition: Specific heat is the amount of heat energy required to raise the temperature of a substance by a certain amount. A high specific heat means that a substance can absorb a large amount of heat energy without its temperature increasing significantly.
- Examples: Water is a common example of a substance with a high specific heat. Other substances with high specific heat include ammonia and liquid hydrogen.
- Importance: High specific heat is important for regulating temperature in organisms and the environment. It helps to stabilize temperature in bodies of water and living organisms, preventing rapid temperature changes that could be harmful.
- Measurement: The specific heat of a substance is typically measured in joules per gram degree Celsius (J/g°C) or calories per gram degree Celsius (cal/g°C).
- Applications: Understanding the concept of high specific heat is important in fields such as biology, environmental science, and engineering. It is also relevant in everyday life, such as in cooking and heating systems.
Understanding the concept of high specific heat and its significance can provide insights into various natural and industrial processes, as well as help in the development of technologies that rely on heat transfer and temperature regulation.
.◂Science Worksheets and Study Guides Kindergarten. Weather
Coloring Worksheet Calendar
Calendar  Coloring Worksheet
Coloring Worksheet Calendar
Calendar  Coloring Worksheet
Coloring Worksheet Day and Night
Day and Night  Coloring Worksheet
Coloring Worksheet Day and Night
Day and Night  Coloring Worksheet
Coloring Worksheet Exploring Weather
Exploring Weather  Coloring Worksheet
Coloring Worksheet Exploring Weather
Exploring Weather  Coloring Worksheet
Coloring Worksheet Look at the Clouds
Look at the Clouds  Coloring Worksheet
Coloring Worksheet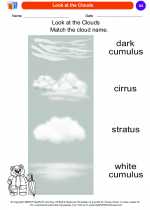 Look at the Clouds
Look at the Clouds  Coloring Worksheet
Coloring Worksheet Moon & Stars
Moon & Stars  Coloring Worksheet
Coloring Worksheet Moon & Stars
Moon & Stars  Coloring Worksheet
Coloring Worksheet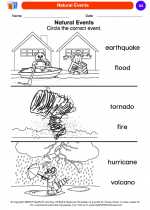 Natural Events
Natural Events  Coloring Worksheet
Coloring Worksheet Natural Events
Natural Events  Coloring Worksheet
Coloring Worksheet Sun and Shadows
Sun and Shadows  Coloring Worksheet
Coloring Worksheet Sun and Shadows
Sun and Shadows  Coloring Worksheet
Coloring Worksheet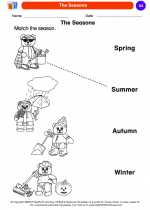 The Seasons
The Seasons  Coloring Worksheet
Coloring Worksheet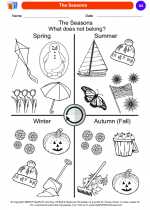 The Seasons
The Seasons  Coloring Worksheet
Coloring Worksheet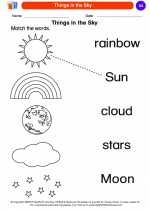 Things in the Sky
Things in the Sky  Coloring Worksheet
Coloring Worksheet Things in the Sky
Things in the Sky  Coloring Worksheet
Coloring Worksheet Weather Tools
Weather Tools  Coloring Worksheet
Coloring Worksheet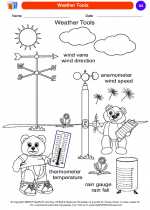 Weather Tools
Weather Tools 

 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet

The resources above cover the following skills:
EARTH AND SPACE SCIENCE (NGSS)
Earth’s Systems
Students who demonstrate understanding can:
Use and share observations of local weather conditions to describe patterns over time.