Universal Solvent
In chemistry, a universal solvent refers to a substance that has the ability to dissolve a wide range of different solutes. The most famous universal solvent is water. Water is often referred to as the "universal solvent" because it has the ability to dissolve a wide variety of substances due to its unique chemical properties.
Why is Water a Universal Solvent?
Water is a polar molecule, meaning it has a slight positive charge on one end and a slight negative charge on the other. This polarity allows water molecules to attract and surround ions and polar molecules, which makes it an excellent solvent for many different substances. Additionally, the structure of the water molecule allows it to form hydrogen bonds with other molecules, further enhancing its ability to dissolve substances.
Study Guide
- What is a universal solvent?
- Give an example of a universal solvent.
- Why is water considered a universal solvent?
- Explain the role of water's polarity in its ability to dissolve substances.
- How do hydrogen bonds contribute to water's ability to dissolve substances?
Understanding the concept of a universal solvent, particularly the properties of water, is important in various fields of science, including chemistry, biology, and environmental science.
.◂Science Worksheets and Study Guides Kindergarten. Weather

 Coloring Worksheet
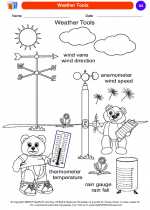
Coloring Worksheet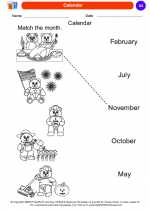
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet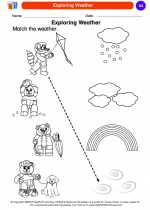
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet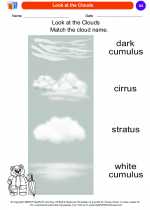
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet