Corrosion
Corrosion is the process of deterioration of materials, usually metals, as a result of chemical reactions with the environment. This can lead to a loss of material and structural integrity, and can be a significant issue in various industries and everyday objects.
Causes of Corrosion
Corrosion can be caused by various factors, including:
- Moisture: Water and humidity can accelerate the corrosion process.
- Oxygen: Exposure to air and oxygen can cause oxidation and corrosion.
- Acids and Bases: Chemicals in the environment can react with metals and cause corrosion.
- Electrolytes: Substances that can conduct electricity, such as saltwater, can accelerate corrosion through electrochemical reactions.
Types of Corrosion
There are several types of corrosion, including:
- Uniform Corrosion: This is the most common type and occurs evenly across the surface of the metal.
- Galvanic Corrosion: This occurs when two different metals are in contact in the presence of an electrolyte, leading to accelerated corrosion of one of the metals.
- Pitting Corrosion: This type creates small holes or pits in the metal, often leading to localized structural damage.
- Crevice Corrosion: Occurs in confined spaces like gaps and crevices where the environment is stagnant, leading to localized corrosion.
- Stress Corrosion Cracking: This occurs under tensile stress and in the presence of a corrosive environment, leading to cracking and failure of the material.
Prevention of Corrosion
There are various methods to prevent or reduce corrosion, including:
- Protective Coatings: Applying paints, plating, or other coatings to create a barrier between the metal and the environment.
- Galvanization: Coating the metal with a layer of zinc to protect it from corrosion.
- Alloying: Mixing the metal with other elements to enhance its corrosion resistance.
- Cathodic Protection: Using sacrificial anodes or impressed current to protect the metal from corrosion.
- Proper Design: Designing objects and structures to minimize areas where moisture can accumulate and to allow for easy inspection and maintenance.
Effects of Corrosion
Corrosion can have significant impacts, including:
- Structural Weakness: Corrosion can compromise the structural integrity of buildings, bridges, and other infrastructure.
- Equipment Failure: Corrosion can lead to the failure of machinery, vehicles, and other equipment.
- Financial Loss: The cost of repairing or replacing corroded materials can be significant for industries and individuals.
- Environmental Impact: Some corrosion products can be harmful to the environment and ecosystems.
Study Guide
To study corrosion effectively, consider the following key points:
- Understand the chemical and electrochemical processes involved in corrosion.
- Learn about the different types of corrosion and their specific characteristics.
- Explore the methods and techniques used to prevent corrosion in various industries and applications.
- Identify real-world examples of corrosion and its impact on structures, equipment, and the environment.
By understanding the causes, types, prevention, and effects of corrosion, you can gain a comprehensive knowledge of this important process and its implications in the world around us.
.◂Science Worksheets and Study Guides Fourth Grade. Cells- The building blocks of living things
Study Guide Cells- The building blocks of living things
Cells- The building blocks of living things  Activity Lesson
Activity Lesson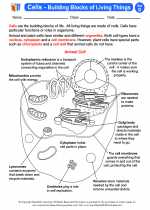 Cells - Building Blocks of Living Things
Cells - Building Blocks of Living Things  Worksheet/Answer key
Worksheet/Answer key Cells- The building blocks of living things
Cells- The building blocks of living things  Worksheet/Answer key
Worksheet/Answer key Cells- The building blocks of living things
Cells- The building blocks of living things  Worksheet/Answer key
Worksheet/Answer key Cells- The building blocks of living things
Cells- The building blocks of living things  Worksheet/Answer key
Worksheet/Answer key Cells - The Building Blocks of Living Things
Cells - The Building Blocks of Living Things  Worksheet/Answer key
Worksheet/Answer key Focus In
Focus In  Vocabulary/Answer key
Vocabulary/Answer key Cells- The building blocks of living things
Cells- The building blocks of living things  Vocabulary/Answer key
Vocabulary/Answer key Cells- The building blocks of living things
Cells- The building blocks of living things  Vocabulary/Answer key
Vocabulary/Answer key Cells- The building blocks of living things
Cells- The building blocks of living things 

 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key

The resources above cover the following skills:
The Living Environment: Students understand that cells are the basic unit of life, that all life as we know it has evolved through genetic transfer and natural selection to create a great diversity of organisms, and that these organisms create interdependent webs through which matter and energy flow. Students understand similarities and differences between humans and other organisms and the interconnections of these interdependent webs.
Cells: Students describe how living things are made up of one or more cells and the ways cells help organisms meet their basic needs.
Give examples of organisms that consist of a single cell and organisms that are made of a collection of cells.